Abstract
Apocrine metaplasia is a benign fibrocystic change characterized by dilated acini lined by columnar cells with apocrine features. These apocrine-like cells form papillary clumps of cells extending into the cystic space called papillary apocrine metaplasia. The researchers report a case of a 48-year-old woman who presented with a palpable mass in the right breast. Breast ultrasonography and MRI showed a large complex cystic and solid mass. A differential diagnosis included intraductal papilloma or intracystic papillary carcinoma. She underwent surgery and the final diagnosis was papillary apocrine metaplasia.
Apocrine metaplasia is a benign epithelial change characterized by dilated acini lined by columnar cells with apocrine cytologic features. The apocrine cytologic features include round, basally located nuclei, abundant granular eosinophilic cytoplasm and apical tufts or snouts at the luminal surface (1). These apocrine-like cells frequently form papillary clumps extending into the cystic space. This common appearance has been termed papillary apocrine metaplasia (2).
Here, we describe a case of papillary apocrine metaplasia presented as a large complex cystic and solid mass mimicking the intraductal papilloma or intracystic papillary carcinoma.
A 48-year-old woman presented with a growing palpable mass in her right breast of three months' duration. The patient didn't have family history of breast cancer nor other malignancy in other organs. She didn't have any history of trauma nor surgery. Physical examination revealed an approximately 5 cm sized mass in the upper portion of the right breast.
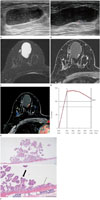
Breast ultrasonography (US) was performed using a HI VISION Ascendus (Hitachi-Aloka Medical, Ltd., Tokyo, Japan) with a 5–18 MHz transducer. On US examination, the lesion presented as an oval, complex cystic and solid mass with circumscribed margin at the 12 o'clock position in the right breast with a maximum diameter of 5.0 × 1.7 cm (Fig. 1A). There was 0.8 cm isoechoic mural nodule and a lot of echogenic debris within the cystic mass. A color Doppler image showed increased blood flow within the mural nodule (Fig. 1B). The lesion was suspicious for papillary neoplasm and classified as a category 4.
The patient underwent breast MRI using a 3.0-T Scanner (GE Discovery MR750w, GE Healthcare, Milwaukee, WI, USA) with a breast coil. The analysis of kinetic enhancement curve patterns was performed using CAD stream TM (Merge Health Care, Chicago, IL, USA). On T2-weighted image, the cystic mass showed bright signal intensity and the mural nodule showed low signal intensity (Fig. 1C). On contrast-enhanced axial image, the mural nodule showed strong contrast enhancement with initial fast and delayed washout pattern (Fig. 1D, E). For pathologic confirmation, a surgical excision was performed. Differential diagnosis was intraductal papilloma or intracystic papillary carcinoma.
On gross examination, the cut surface of the specimen showed a unilocular cyst containing watery materials. Microscopic examination (Fig. 1F) revealed a unilocular cyst lined with a single layer of columnar cells with apocrine feature. There was a papillary mass measuring 1 × 0.8 × 0.3 cm, which was composed of columnar cells with apocrine feature. The final histologic diagnosis was papillary apocrine metaplasia.
Apocrine metaplasia is a benign fibrocystic change. Benign proliferative lesions with apocrine metaplasia have been considered not to have increased risk of breast cancer development (3). In the study of Page et al. (2), when the papillary apocrine metaplasia occurred without proliferative disease, there was no increased risk of breast cancer. When the papillary apocrine metaplasia occurred with concurrent foci of proliferative lesion, there was slight elevation of breast cancer risk (at least 1.5 times).
Apocrine metaplasia commonly appeared as clustered microcysts. Warner et al. (4) reported that 10 (77%) of the 13 sonographically visible lesions of apocrine metaplasia having this appearance. Although adjacent acini in the apocrine metaplasia could fuse and form larger cysts over time (5), a previous study reported that such changes occurred in only two (3%) of 66 clustered microcysts during more than 2 years (6). Not only the size of cyst, also the size and number of internal mural nodules could increase in papillary apocrine metaplasia (7).
In the study of Kushwaha et al. (8), although no specific finding was not detected on mammography, many lesions (73%) appeared as new or increased calcifications with coarse heterogeneous, amorphous or punctate shapes. On MRI, 17 of 19 (89%) papillary apocrine metaplasia were 10 mm or smaller and 14 (74%) lesions were at least partially high signal intensity on T2WI (9). Eleven (58%) lesions showed washout kinetics. Also in our case, the cystic mass was high signal intensity on T2WI and the mural nodule showed strong contrast enhancement with initial fast and delayed washout pattern.
In our case, the papillary apocrine metaplasia was presented as a large complex cystic and solid mass mimicking intraductal papilloma or intracystic papillary carcinoma. In case of intraductal papilloma or papillary carcinoma, the cyst wall to which mural nodule attaches will appear irregular and there will be ductal extension because the papilloma or carcinoma extends to the draining duct causing distension. However, in case of apocrine metaplasia, the site of attachment to the cystic wall remains preserved and mural nodules do not extent into the ducts, causing no obstruction of adjacent ducts (10). In our case, there was no irregularity in the attached wall and no adjacent ductal distention favoring the papillary apocrine metaplasia. We also supposed that relatively small size of mural nodule compared to a large cyst could be the clue for the diagnosis of papillary apocrine metaplasia.
In conclusion, we report a case of papillary apocrine metaplasia manifested as large complex cystic and solid mass. Despite its rarity, if radiologists recognize radiographic findings, the possibility of papillary apocrine metaplasia should be considered as a differential diagnosis. It could be helpful in making appropriate treatment plan and follow up.
Figures and Tables
Fig. 1
A 48-year-old woman with papillary apocrine metaplasia.
A. Breast sonography shows an oval, complex cystic and solid mass with internal echogenic debris in the 12 o'clock direction of the right breast.
B. Increased blood flow within the mural nodule is noted on color Doppler image.
C. On axial fat-saturated T2-weighted image, the cystic mass shows bright signal intensity and the mural nodule shows low signal intensity.
D. On dynamic contrast-enhanced study, the mural nodule shows strong contrast enhancement.
E. Kinetic curve pattern is initial fast and delayed washout pattern.
F. Microscopic examination shows a unilocular cyst lined with a single layer of columnar cells with apocrine feature (arrow) and a small papillary projection of apocrine-like cells (thick arrow) (hematoxylin and eosin stain, × 12.5 and × 100).
References
1. Durham JR, Fechner RE. The histologic spectrum of apocrine lesions of the breast. Am J Clin Pathol. 2000; 113:5 Suppl 1. S3–S18.

2. Page DL, Dupont WD, Jensen RA. Papillary apocrine change of the breast: associations with atypical hyperplasia and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1996; 5:29–32.
3. Godfrey SE. Is fibrocystic disease of the breast precancerous? Arch Pathol Lab Med. 1986; 110:991.
4. Warner JK, Kumar D, Berg WA. Apocrine metaplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1998; 170:1375–1379.

5. Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975; 55:231–273.
6. Berg WA. Sonographically depicted breast clustered microcysts: is follow-up appropriate? AJR Am J Roentgenol. 2005; 185:952–959.

7. Ferguson MJ. Intracystic mural nodules of the breast: benign versus malignant; a multidisciplinary imaging and management approach. J Med Radiat Sci. 2015; 62:163–167.

8. Kushwaha AC, O'Toole M, Sneige N, Stelling CB, Dryden MJ. Mammographic-pathologic correlation of apocrine metaplasia diagnosed using vacuum-assisted stereotactic core-needle biopsy: our 4-year experience. AJR Am J Roentgenol. 2003; 180:795–798.
9. diFlorio-Alexander RM, Marotti JD, Bond JS, Schwab MC, Memoli VA, Wells WA, et al. Breast MRI-detected cystic apocrine metaplasia: imaging features with microvessel analysis and histologic correlation. AJR Am J Roentgenol. 2015; 204:211–218.

10. Stavros AT, Rapp CL, Parker SH. Breast ultrasound. Philadelphia, PA: Lippincott Williams & Wilkins;2004.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download