Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a complex heterogeneous condition with various clinical and pathologic features. In recent years, technical advances in quantitative CT imaging have generated considerable interest because they can provide a more precise and objective assessment of COPD. Emphysema and small-airway disease, the two major components of COPD, and other comorbidities, including pulmonary vessel alterations, atherosclerosis, cachexia, and osteoporosis, can all be assessed by means of quantitative imaging parameters. Increasing numbers of studies provide promising reports indicating that such parameters are associated with clinical measures of disease severity, respiratory symptoms, COPD exacerbations, and mortality. Despite such optimistic results, there are still many obstacles to using this quantitative technology in everyday practice to manage COPD patients. In this article, we review the current technical status of quantitative CT assessment, emphasizing its clinical implications and limitations. We also discuss present challenges and the potential future role of quantitative CT imaging in assessing COPD.
Chronic obstructive pulmonary disease (COPD) is defined as a common preventable and treatable disease characterized by persistent airflow limitation, which is usually progressive, and is associated with enhanced chronic inflammatory responses in the airways and lung (1). COPD is a major cause of morbidity and mortality in the general population, and the WHO estimates that it will become the third leading cause of death worldwide (2). It is a heterogeneous and complex condition, with a wide variety of clinical and pathologic characteristics. Even though pulmonary function test (PFT) parameters are currently used to diagnose and classify the severity of COPD, they cannot fully represent the type and range of the heterogeneous pathophysiologic abnormalities present in COPD. Additionally, the PFT tends to be relatively insensitive to early stages and subtle changes in COPD, before functional parameters begin to show changes (3). Furthermore, patients with similar PFT values may demonstrate completely different clinical and radiological phenotypes.
In the era of precision medicine, a more meticulous understanding of clinical disease manifestation and its relationship with endogenous disease pathogenesis will likely have significant implications on disease management and understanding of the disease process. Chest CT is the most commonly used imaging modality; it is essentially non-invasive and provides information regarding the structural and underlying pathophysiologic changes in the COPD patient. CT scans allow in vivo visual analysis of morphologic characteristics and the distribution of both emphysema and small airway disease, the two main components of COPD. However, qualitative visual CT assessment, which has been the mainstream method for acquiring information from CT scans, is prone to inter-reader, and sometimes even intra-reader, variability, limiting its application in broad clinical and experimental settings (4). Therefore, the need for more objective CT-based measures has grown significantly over the past decade. In this context, recent technical advances in quantitative CT imaging methods are of considerable interest for their ability to provide more precise and reproducible estimates of the severity and distribution of emphysema and airway disease (5). In the early days, research on the quantification of CT in COPD was weighted towards quantification of emphysema. However, there have recently been an increasing number of publications targeting the airway component of COPD, and these can be further divided into direct airway parameter measurements and quantification of air trapping as the functional manifestation of small airway disease. In this review, we briefly address previous studies on COPD quantification, highlight the current concepts, and discuss the future direction of quantified CT imaging.
Pulmonary emphysema, defined as an abnormal irreversible dilatation of air spaces and destruction of airway walls distal to terminal bronchioles, is a major finding in COPD. Due to the increased proportion of air compared with normal lung parenchyma, emphysema appears as a region of relatively lower CT attenuation, expressed as lower Hounsfield units (HU). The density mask method, first introduced in 1988, quantifies emphysema by calculating the voxels that are lower than a certain HU threshold, referring to such voxel regions as low attenuation areas (LAA) (6). The HU threshold of -910 was initially proposed in 1988 and various different threshold values have been tested afterwards. In 2006, Madani et al. (7) has shown the optimal HU threshold to be -960 HU or -970 HU according to macroscopic and microscopic pathologic correlations. However, because higher threshold values result in higher sensitivity to image noise, the most commonly accepted threshold value is -950 HU (8), with the percentage area of lung less than -950 HU (the emphysema index, or %LAA-950) being widely used to estimate the emphysema component in COPD patients (Fig. 1A, B) (9). An alternative approach, based on the frequency histogram of lung attenuation, calculates the lung parenchymal HU at a given percentile along the histogram. This value is called the “percentile index,” and validation with pathologic specimens has shown that the optimal threshold is the first percentile (7). However, because of concerns regarding increased image artifacts and noise at the first percentile level, the 15th percentile threshold is most commonly used (810). The percentile index has been reported to be more robust for evaluating longitudinal variations in emphysema, and as being less sensitive to lung volume changes (811).
An innate limitation of CT histogram measurement approaches is that they do not take into account the heterogeneous patterns of distribution, size, or clusters of emphysema, which could be influential factors in the evaluation of a COPD patient. Since emphysema is a regionally distributed disease, determination of the anatomic zonal distribution of emphysema, rather than simple quantification of the total amount of emphysematous involvement, may be of importance. Most currently available quantitative CT methods are able to divide each lung into separate anatomical zones, allowing ratios of the extent of emphysema in different lung zones to be calculated. More recent methods also permit the segmentation of lobes to measure lobar volumes and the extent of emphysema objectively (Fig. 1C). To quantify the local size of lung parenchyma showing emphysematous changes, several investigators have used low attenuation cluster analysis by determining the power-law exponents (D) of emphysema holes; this represents the cumulative frequency-size distribution of the emphysema, and estimates how the emphysema areas are conglomerated to form small-to-large “clustered” regions of emphysema (121314).
Quantification of emphysema is relatively simple, and quantitative CT measurements have been shown to correlate better with macroscopic measurements of emphysema than do measures of visual CT assessment (15). In patients with α1-antitrypsin deficiency, density-based emphysema measurement has shown a superior accuracy in detecting worsening of emphysema, and therefore has been accepted as the effective diagnostic method for detecting disease progression (16). The CT density mask method was found to have an association with COPD mortality, and is reported to be a stronger predictive parameter for cardiac and respiratory mortality than global initiative for chronic obstructive lung disease (GOLD) staging (1718). Patients with greater quantitative measures of emphysema were reported to have more rapid decline of PFT parameters (19). Furthermore, quantified measures of emphysema showed associations with frequent exacerbation and worse clinical outcomes from exacerbation events (202122). When the heterogeneity of emphysema assessed using quantitative CT was taken into account, the basal distribution of emphysema was associated with greater impairment in forced expiratory volume in 1 second (FEV1), with central areas of emphysema showing a stronger correlation with airflow limitation than those in the periphery of the lung (2324). In one report, the quantitatively measured heterogeneity of emphysema distribution was associated with a decrease in FEV1 and FEV1/forced vital capacity (FVC) (25). When compared with the %LAA-950 value obtained from the whole lung, histogram-based measures of different patterns of emphysema involvement were more predictive of measures of PFT results, severity of dyspnea, and quality of life (26). Additionally, the regional distribution of emphysema has been shown to be clinically important in selecting optimal candidates for lung volume reduction surgery (27).
Small airway remodeling is an important factor in COPD, and is known to be the strongest determinant of airflow limitation. A small airway is defined as an airway with an internal diameter smaller than 2 mm, and because of the limited spatial resolution of even the most up-to-date CT scanners, the accurate direct measurement of small airway dimensions has proven to be a great challenge. Current quantitative CT techniques allow three dimensional reconstruction of the airways and adequate non-invasive measurement of segmental and sub-segmental airways, which are “large airways” by definition (Fig. 2A). However, a previous report showed that changes in large airways reflect changes in small airways on pathologic examination, and the measured CT parameters of large airways correlated with PFT results (28). Additionally, the measured CT parameters of more distal airways showed stronger correlations with spirometry (29). Therefore, it may be of value to obtain quantified airway parameters from measurable large airways on CT of COPD patients (Fig. 2B).
The most widely used methodology for obtaining quantitative airway measurements is the “full-width half-maximum (FWHM)” method. This uses projecting linear rays from the airway center to determine the inner and outer margin of the airway wall, using an HU threshold for differentiating the wall from the surrounding lung parenchyma (Fig. 3A). It is a simple and straightforward method that has been incorporated into various commercially available software, but the risk of overestimation of wall parameters is a well-known limitation, which becomes more obvious as the measured airway becomes smaller in size (30). A variety of other algorithms have been developed and tested, including a threshold-based method that showed better correlation with PFT than the FWHM method (31), but there is currently neither consensus nor solid evidence for validation of the most accurate and standardized method of airway measurement in COPD (323334). Diverse airway parameters can be obtained from CT, including airway wall thickness, wall area, lumen diameter, lumen area, wall area percentage (WA%), and internal perimeter (Fig. 3B). However, quantitative parameters of the airway may be influenced by the different anatomical locations within the airway tree where the measurements are made, resulting in difficulties comparing the measurements between research groups. Recently, another CT airway parameter called Pi10 was introduced to avoid the potential bias that may occur due to the different distribution of airway sizes. It is derived by plotting the square root of the airway wall area against the internal perimeter of each measured airway, and using the regression line to calculate the square root of the airway wall area for a hypothetical representative airway with an internal perimeter of 10 mm (35). Pi10 provides a method for standardizing airway measurements for comparisons between subjects.
Increased bronchial wall thickness and WA% is reported to be correlated with deterioration of FEV1, and this correlation is reported to be stronger with more distal airways (29313236). Also, in subjects with a comparatively low emphysema component, quantitative CT measurements of airways showed stronger correlations with physiologic indices (37). Airway parameters also correlated with functional markers and exercise capacity of COPD patients (938). It is hypothesized that increased bronchial wall thickness may be closely related to inflammation of the airway, and thus may have an association with aggravated symptoms of bronchitis or exacerbations. Accordingly, increased quantitative parameters of airway wall thickness were related to an increased frequency of, and mortality from, COPD exacerbations (2021). However, compared with emphysema quantification, there are still many technical difficulties and uncertainties regarding measurements of airway changes in COPD patients, and further investigation is warranted.
As previously mentioned, airways with a diameter less than 2 mm are the major sites of airflow obstruction, and small airway narrowing is known to occur in early stages of COPD, before major emphysematous changes occur (39). Small airway wall remodeling is known to be the most dominant factor for the airflow limitation observed in COPD patients, and small airway changes occur due to abnormal cellular proliferation and peribronchial fibrosis. As CT is unable to adequately image the small airways directly because of its limited resolution, many researchers have used different parameters of air trapping measured on expiratory phase CT as functional estimates of small airway disease.
One of the earliest and simplest ways to estimate air trapping using chest CT is to evaluate the percentage of low attenuation area at a threshold of -856 HU or -850 HU. The threshold is chosen because it is known to be the attenuation of normally inflated inspiratory lung, with the concept being that healthy expiratory lung should show higher attenuation than this value. Using this relatively straightforward method, several researchers have reported high correlations with spirometry results, including FEV1/FVC and FEV1 percent predicted (340). Other methods for measuring air trapping have been addressed, using information from both inspiratory and expiratory CT, including the ratio of inspiratory to expiratory lung volume and the expiratory to inspiratory ratio of mean lung density.
However, the above methods have an innate limitation, in that it is impossible to separate trapped air from emphysematous lung or obstructed small airways. One method developed to quantify air trapping outside the emphysematous area is measurement of the relative volume change between -860 HU and -950 HU (41). This method excludes the emphysema portion of all voxels with attenuation lower than -950 HU from the inspiration and expiration scans, and calculates for the whole lung the relative volumes with attenuation values less than -860 HU on each inspiratory and expiratory CT. Results from this method revealed strong associations with physiologic parameters of gas trapping; however, this method was limited by the fact that pixel-by-pixel matching of inspiratory and expiratory scans was not performed.
To overcome such a limitation, a new quantitative method called parametric response mapping has recently been developed; this involves pixel-by-pixel co-registration of inspiratory and expiratory CT scans so that a more exact comparison can be obtained (5). This method enables the classification of emphysema and functional small airway disease, and is reported to show an increased correlation with PFT results (42). Another method, called air trapping index (ATI), relies on the HU difference of each of the image voxels detected on co-registered inspiratory and expiratory CT scans (Fig. 4) (4344). Unlike other methods, both parametric response mapping and ATI methods allow additional assessment of the regional heterogeneous distribution of air trapping.
Quantified parameters of air trapping were found to be associated with changes in FEV1 and other clinical parameters, and with aggravating GOLD classification (4145). Additionally, results from paired evaluations of inspiratory and expiratory CT images revealed that they were stronger predictors of spirometry results than the use of just the expiratory scan (46). Quantitatively measured air trapping parameters in a large cohort study were associated with FEV1 deterioration, which was notably prominent in patients with a lesser severity of COPD (47). Although air trapping parameters provide assessment of only the consequence of pathological airway changes, such techniques contribute to the characterization of disease phenotypes, and considerable research is ongoing.
Pulmonary vascular change is a prevalent feature of COPD and is reported to be a strong predictor of mortality. However, the actual pathogenesis of pulmonary vascular changes in COPD is still a vastly unknown territory, and new quantitative CT methods are being developed to better understand the relationship between vascular changes and other components of COPD. The pulmonary artery diameter and the ratio of the pulmonary artery diameter to aorta diameter were associated with pulmonary artery pressure and increased exacerbation rates (48). When the cross sectional area (CSA) of the pulmonary arteries was measured using quantitative volumetric CT, total CSA showed a strong correlation with the severity of emphysema, and a significant but lesser degree of correlation with air trapping in COPD patients (49). Using a technique to automatically segment pulmonary vasculature and measure the total blood volume, pruning of distal arteries was suggested as an early change in smoking-related COPD, with the extent of emphysema having a negative relationship with total pulmonary blood volume (50). The clinical significance of these findings still needs to be clarified, and continued investigation is much needed.
Ischemic heart disease is a common comorbidity in COPD. Patients with COPD are prone to more frequent and more fatal myocardial infarctions, even after adjusting for common risk factors such as smoking status and old age (51). Furthermore, reduced FEV1 has been reported to be an increased risk factor for cardiovascular mortality (52). The quantitative measurement of coronary artery calcium (CAC) is an accurate and non-invasive parameter of coronary artery atherosclerosis. A high CAC score is reported to be an independent predictor of future cardiovascular events, and COPD patients were found to have higher CAC scores and increased cardiovascular mortality (53). In addition, calcium score used as a measurement of the degree of systemic atherosclerosis was reported to have a weak but significant correlation with the volume fraction of emphysema on quantitative CT, FEV1/FVC, and diffusion capacity, with these correlations being independent of age, body mass index (BMI), and smoking amount (54).
Cachexia and skeletal muscle wasting are common comorbidities in COPD patients, and changes in body composition are reported to be associated with COPD severity, airflow obstruction, and mortality. Thoracic respiratory muscles in particular are unique and crucial for alveolar ventilation, and a weakness of respiratory muscle results in the dyspnea and respiratory failure associated with mortality in COPD patients. One group quantitatively measured the area of pectoralis muscle using axial CT images and revealed a significantly decreased muscle area in COPD patients compared with healthy individuals, with patients having a decreased muscle area generally having a worse disease stage. Decreased pectoralis muscle area was also associated with functional markers of disease, including BODE index, dyspnea score, and other parameters of patient activity (55). In a similar context, the measurements of intercostal muscle mass and attenuation using quantitative CT methods were significantly correlated with COPD severity and the quantitatively assessed extent of emphysema on CT (56). A decrease in thoracic muscle mass with increasing intercostal fat was associated with worsening of COPD severity.
COPD patients are at a higher risk of osteoporosis because the two entities share similar clinical factors such as smoking, decreased physical activity, low BMI, prolonged steroid use, and vitamin D deficiency. Furthermore, osteoporosis often results in vertebral compression fractures, which can deteriorate FEV1 and decrease vital capacity in COPD patients. Although dual-energy X-ray absorptiometry of the lumbar spine and hip is currently the gold standard for the diagnosis of osteoporosis, quantitative CT has recently emerged as a solid alternative for assessing bone mineral density (BMD), using a three dimensional technique to provide volumetric assessment of bone. In a recent report, COPD, especially the emphysema dominant type, was associated with an increased severity of osteoporosis measured on chest CT, even after adjustment for conventional risk factors (57). Moreover, decreased thoracic vertebral BMD was an independent predictor of higher rates of exacerbations and other morbidity measures in COPD patients (58).
Although quantitative analysis of CT has shown promising results, further work is required to implement quantitative CT parameters as imaging biomarkers that can be used to practically manage COPD patients. First, methods for quantifying the various components of COPD need standardization, optimization, and simplification. Although various methods have shown good correlations with physiologic or clinical parameters, there is currently neither consensus nor a gold standard method for quantifying each component of COPD. Furthermore, quantitative parameters derived from CT images can be influenced by both patient and CT-related factors, including patient weight, inspiration adequacy, CT manufacturer, and calibration. Optimization of CT protocols and quality control in image acquisition are critical for large multicenter/multinational trials and comparisons of results from different subjects. Additionally, one very important practical point is that the time and effort required for quantitative assessment, including post-processing procedures, should be minimized, with procedures being automated to improve their clinical accessibility and utility.
The present GOLD strategy does not recommend routine use of CT scanning in COPD, and only advises that it may be helpful in excluding differential diagnosis or when surgical options, such as lung volume reduction surgery, are being considered. This is largely because of a relative lack of evidence that information gathered from CT can actually modify treatment plans, predict treatment response or prognosis, and eventually alter the mortality of COPD patients. A large number of prior studies have compared quantitative CT parameters with simple PFT results, and more analyses need to be assessed against disease outcome measures, effect of treatment, and other clinically significant markers of disease severity. Similarly, investigation of the relationship between genetic information and quantitative imaging parameters in COPD could provide crucial information on disease pathogenesis because individual manifestations of COPD may vary according to genetic variations. Additionally, more longitudinal cohort studies are required to track CT changes over time and obtain an accurate picture of disease progression and how it can be effectively captured by quantitative CT imaging. Further work should be performed to analyze the clinically important COPD phenotypes using quantitative CT techniques because such information may have an important influence on COPD trials and drug development, which are in great clinical demand.
Lastly, quantitative CT analysis should be augmented with the rapidly increasing number of new up-to-date technologies. Dose reduction technology, such as low dose protocols, noise lowering procedures, and new image reconstruction algorithms, are vital for longitudinal studies and the more widespread use of CT in clinical practice. Dual-energy CT technology allows functional imaging of the lung and may have further applications in quantitative evaluation of COPD patients. In the era of big data analysis, radiomics is a recent active field of study, in which high throughput data is extracted and a large amount of advanced quantitative imaging features are analyzed, often automatically or semi-automatically. Although most published radiomics studies have largely focused on cancer research, application of radiomics to COPD may reveal additional insights. Artificial intelligence, including deep learning and machine learning technology, although still a vastly unknown field of study, has also gained great worldwide attention in the past few years, and is another potential field of study that could be augmented by quantitative CT technology.
Quantitative CT imaging is a promising technique for objectively measuring disease manifestation, and has already shown associations with traditional clinical and physiologic markers of COPD. Quantitative CT analysis provides reliable estimates of two dominant features of COPD, emphysema and airway disease, and various other features, including pulmonary vessel alterations, atherosclerosis, cachexia, and osteoporosis. These quantified parameters can be used as important research tools to understand the underlying heterogeneity of COPD, and to assist in revealing the fundamental pathogenesis in it. The translation of information derived from these approaches into actual clinical practice has the potential to guide individualized management strategies and improve disease outcomes in COPD patients.
Figures and Tables
 | Fig. 1Quantitative CT measurement of emphysema.
A. Visual assessment of coronal reconstructed CT image of a patient with chronic obstructive pulmonary disease reveals emphysema involvement with upper lobe dominancy.
B. Using the density mask method with a threshold of -950 HU, areas with HU values lower than the threshold (low attenuation areas%) can be readily quantified, and overlaying of the density mask (shown in green on online figure) allows a more robust assessment of emphysema.
C. Most available CT quantification software provides reliable automatic segmentation of the lung, making regional quantification of emphysema possible.
HU = Hounsfield unit
|
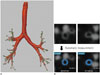 | Fig. 2Quantitative assessment of airways.
A. Recent technical advances allow more accurate and robust automatic extractions of airways, with three dimensional volumetric reconstructions.
B. Once airways are extracted and target points in the airways are selected, quantitative airway parameters are automatically measured (shown in blue on online figure).
|
 | Fig. 3Full-width at half-maximum method and commonly measured airway parameters.
A. In the attenuation profile along an outwards flowing ray from the luminal center-point through to the airway wall, the inner and outer airway wall boundaries are assumed halfway to the maximum on the lumen side, and halfway to the minimum on the parenchymal side, respectively.
B. Diverse airway parameters can be obtained using quantitative analysis, including wall thickness WA, WT, LA, LD, Pi, and WA%. HU = Hounsfield units, LA = lumen area, LD = lumen diameter, Pi = internal perimeter, WA = wall area, WA% = wall area percentage, WT = wall thickness
|
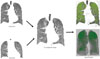 | Fig. 4Air trapping measurement using a co-registration method. Using an image co-registration technique, expiratory CT images are modified and matched with inspiratory CT images. This technique allows voxel-by-voxel comparisons of attenuation changes between inspiration and expiration, with air trapping being defined as areas with less change in attenuation than the preset threshold (60 Hounsfield units in the example shown above). |
Acknowledgments
This work was supported by the Technological Innovation R&D Program (S2464035) funded by the Small and Medium Business Administration (SMBA, Korea).
References
1. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.
2. Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006; 27:397–412.

3. Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013; 201:W460–W470.

4. Cavigli E, Camiciottoli G, Diciotti S, Orlandi I, Spinelli C, Meoni E, et al. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol. 2009; 19:1686–1692.

5. Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012; 18:1711–1715.

6. Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988; 94:782–787.
7. Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology. 2006; 238:1036–1043.

8. Heussel CP, Herth FJ, Kappes J, Hantusch R, Hartlieb S, Weinheimer O, et al. Fully automatic quantitative assessment of emphysema in computed tomography: comparison with pulmonary function testing and normal values. Eur Radiol. 2009; 19:2391–2402.

9. Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008; 186:157–165.

10. Stolk J, Dirksen A, van der Lugt AA, Hutsebaut J, Mathieu J, de Ree J, et al. Repeatability of lung density measurements with low-dose computed tomography in subjects with alpha-1-antitrypsin deficiency-associated emphysema. Invest Radiol. 2001; 36:648–651.
11. Dirksen A. Monitoring the progress of emphysema by repeat computed tomography scans with focus on noise reduction. Proc Am Thorac Soc. 2008; 5:925–928.

12. Gietema HA, Müller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, et al. Quantifying the extent of emphysema: factors associated with radiologists' estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol. 2011; 18:661–671.
13. Hwang J, Lee M, Lee SM, Oh SY, Oh YM, Kim N, et al. A size-based emphysema severity index: robust to the breath-hold-level variations and correlated with clinical parameters. Int J Chron Obstruct Pulmon Dis. 2016; 11:1835–1841.
14. Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999; 96:8829–8834.

15. Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999; 211:851–858.

16. Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999; 160(5 Pt 1):1468–1472.
17. Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010; 138:635–640.

18. Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013; 187:602–608.

19. Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011; 66:782–787.

20. Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011; 261:274–282.

21. Jairam PM, van der Graaf Y, Lammers JW, Mali WP, de Jong PA. PROVIDI Study group. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax. 2015; 70:725–731.

22. Oh YM, Sheen SS, Park JH, Jin UR, Yoo JW, Seo JB, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis. 2014; 18:1407–1414.

23. Nakano Y, Sakai H, Muro S, Hirai T, Oku Y, Nishimura K, et al. Comparison of low attenuation areas on computed tomographic scans between inner and outer segments of the lung in patients with chronic obstructive pulmonary disease: incidence and contribution to lung function. Thorax. 1999; 54:384–389.

24. Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004; 170:1172–1178.
25. Chae EJ, Seo JB, Song JW, Kim N, Park BW, Lee YK, et al. Slope of emphysema index: an objective descriptor of regional heterogeneity of emphysema and an independent determinant of pulmonary function. AJR Am J Roentgenol. 2010; 194:W248–W255.

26. Castaldi PJ, San José R, Mendoza CS, Hersh CP, Laird N, Crapo JD, et al. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013; 188:1083–1090.

27. Nakano Y, Coxson HO, Bosan S, Rogers RM, Sciurba FC, Keenan RJ, et al. Core to rind distribution of severe emphysema predicts outcome of lung volume reduction surgery. Am J Respir Crit Care Med. 2001; 164:2195–2199.

28. Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005; 171:142–146.

29. Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006; 173:1309–1315.

30. Kim N, Seo JB, Song KS, Chae EJ, Kang SH. Semi-automatic measurement of the airway dimension by computed tomography using the full-with-half-maximum method: a study of the measurement accuracy according to the orientation of an artificial airway. Korean J Radiol. 2008; 9:236–242.

31. Cho YH, Seo JB, Kim N, Lee HJ, Hwang HJ, Kim EY, et al. Comparison of a new integral-based half-band method for CT measurement of peripheral airways in COPD with a conventional full-width half-maximum method using both phantom and clinical CT images. J Comput Assist Tomogr. 2015; 39:428–436.

32. Achenbach T, Weinheimer O, Biedermann A, Schmitt S, Freudenstein D, Goutham E, et al. MDCT assessment of airway wall thickness in COPD patients using a new method: correlations with pulmonary function tests. Eur Radiol. 2008; 18:2731–2738.

33. Berger P, Perot V, Desbarats P, Tunon-de-Lara JM, Marthan R, Laurent F. Airway wall thickness in cigarette smokers: quantitative thin-section CT assessment. Radiology. 2005; 235:1055–1064.

34. King GG, Müller NL, Whittall KP, Xiang QS, Paré PD. An analysis algorithm for measuring airway lumen and wall areas from high-resolution computed tomographic data. Am J Respir Crit Care Med. 2000; 161(2 Pt 1):574–580.

35. Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009; 34:858–865.

36. Yamashiro T, Matsuoka S, Estépar RS, Dransfield MT, Diaz A, Reilly JJ, et al. Quantitative assessment of bronchial wall attenuation with thin-section CT: an indicator of airflow limitation in chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2010; 195:363–369.

37. Nambu A, Zach J, Schroeder J, Jin G, Kim SS, Kim YI, et al. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur J Radiol. 2016; 85:2144–2151.

38. Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, Curtis JL, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012; 67:399–406.

39. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011; 365:1567–1575.

40. Murphy K, Pluim JP, van Rikxoort EM, de Jong PA, de Hoop B, Gietema HA, et al. Toward automatic regional analysis of pulmonary function using inspiration and expiration thoracic CT. Med Phys. 2012; 39:1650–1662.

41. Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008; 190:762–769.

42. Barbosa EM Jr, Song G, Tustison N, Kreider M, Gee JC, Gefter WB, et al. Computational analysis of thoracic multidetector row HRCT for segmentation and quantification of small airway air trapping and emphysema in obstructive pulmonary disease. Acad Radiol. 2011; 18:1258–1269.
43. Kim EY, Seo JB, Lee HJ, Kim N, Lee E, Lee SM, et al. Detailed analysis of the density change on chest CT of COPD using non-rigid registration of inspiration/expiration CT scans. Eur Radiol. 2015; 25:541–549.

44. Lee SM, Seo JB, Lee SM, Kim N, Oh SY, Oh YM. Optimal threshold of subtraction method for quantification of air-trapping on coregistered CT in COPD patients. Eur Radiol. 2016; 26:2184–2192.

45. Rambod M, Porszasz J, Make BJ, Crapo JD, Casaburi R. COPDGene Investigators. Six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest. 2012; 141:867–875.

46. Hersh CP, Washko GR, Estépar RS, Lutz S, Friedman PJ, Han MK, et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res. 2013; 14:42.

47. Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016; 194:178–184.
48. Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012; 367:913–921.

49. Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010; 17:93–99.
50. Estépar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013; 188:231–239.

51. Stefan MS, Bannuru RR, Lessard D, Gore JM, Lindenauer PK, Goldberg RJ. The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction: a 10-year retrospective observational study. Chest. 2012; 141:1441–1448.
52. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005; 127:1952–1959.
53. Williams MC, Murchison JT, Edwards LD, Agustí A, Bakke P, Calverley PM, et al. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014; 69:718–723.

54. Chae EJ, Seo JB, Oh YM, Lee JS, Jung Y, Lee SD. Severity of systemic calcified atherosclerosis is associated with airflow limitation and emphysema. J Comput Assist Tomogr. 2013; 37:743–749.

55. McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014; 11:326–334.

56. Park MJ, Cho JM, Jeon KN, Bae KS, Kim HC, Choi DS, et al. Mass and fat infiltration of intercostal muscles measured by CT histogram analysis and their correlations with COPD severity. Acad Radiol. 2014; 21:711–717.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download