Dear Editor:
Galectins are a family of soluble β-galactoside-binding lectins that share a unique carbohydrate recognition domain. Among the various galectins, galectin-3 and galectin-7 are of great interest in the field of cutaneous biology. These galectins are found abundantly in the cytoplasm of epidermal keratinocytes and are associated with cutaneous wound healing through the promotion of reepithelialization1.
Given the relationship between wound healing process and galectins, we studied the expression patterns of galectin-3 and -7 in human skin of different ages and two distinct skin equivalent (SE) models. Because aging affects the wound healing and repair processes, we assume that their expression patterns vary with respect to age and characteristics of SEs.
Nine healthy individuals of different ages (6~80 years old) were enrolled. All skin samples were from the non-exposed areas (buttocks) to rule out the effects of ultraviolet exposure on galectin expression2. Additionally, two different SE models were reconstructed according to the method as described previously3. Human keratinocytes and fibroblasts were isolated from human foreskins obtained during circumcision. Keratinocytes were cultured in two distinct dermal equivalents, one prepared by standard stock solutions and the other by standard stock solutions and adjuvants (hyaluronic acid and Cervi cornus Colla). We previously reported that these adjuvants are helpful by enhancing the formation of epidermis and basement membranes3. This study was approved by Seoul National University Bundang Hospital Institutional Review Board (IRB no. 1305/202-004 and 1207/164-003).
Immunohistochemical staining was performed on paraffin sections. For immunofluorescence study, the sections were incubated overnight at 4℃ with an antibody directed against galectin-3 (B2C10; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and galectin-7 (G-3; Santa Cruz Biotechnology). After washing, the samples were incubated with secondary antibodies for 2 hours and then stained with 4,6-diamino-2-phenylindole (DAPI) at 1 µg/ml for 10 minutes. In confocal microscope images, galectin-3 and -7 were shown as the green and the red color, respectively. Cell nuclei were also counterstained with DAPI. For each sample on a slide, three non-overlapping areas with similar cell numbers were captured with the same magnification. Photomicrographs were acquired using the same illumination and exposure time for every section. Image analysis was performed in the two areas of interest, whole epidermis and the basal layer using Image Pro-Plus 6.0 (MediaCybernetics Inc., Rockville, MD, USA). To quantify the galectin expression of the area, we used the intensity analysis methods previously described by Arqués et al.4. The signal intensity was calculated and noted as relative units (RUs).
In immunofluorescence images, galectin-3 and -7 were mainly stained in the cytoplasm rather than the nucleus of the cells. These galectins were observed throughout the epidermis, and staining intensity became more intense in the upper layers of the epidermis. With respect to age, fluorescence intensities of both galectins were stronger in young skin than in old skin. In the dermis, there were scattered foci of positive staining of galectin-3; however, galectin-7 was not detected at all (Fig. 1).
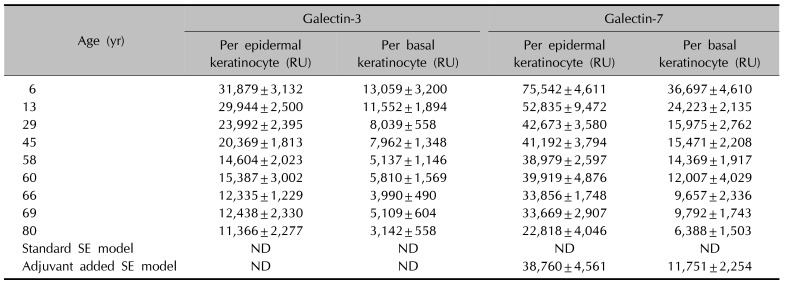
The level of expression for galectin-3 (or galectin-7) per keratinocyte in the epidermis were calculated as shown in Table 1. Data represent the mean RU±standard deviation from three different fields of each sample slide. In normal skin, RUs of basal keratinocytes were as low as about one-half to one-third that of epidermal keratinocytes. This suggests that there were more galectin expressions as there were more differentiated keratinocytes. In addition, RUs of both galectin-3 and -7 also decreased with aging. Decreasing trends of RUs were similar for both galectins. In immunofluorescence images of SEs, both models of SEs were not stained with galectin-3. However, adjuvant added SEs were strongly stained with galectin-7 (Fig. 1), and the expression patterns of galectin-7 in SEs were similar to those of human skin samples in 60s (Table 1).
Our results showed that there was an age-dependent decrease in the level of galectin-3 and -7 expressions in keratinocytes and basal keratinocytes. Recent studies revealed a close association of galectin-3 and -7 with the cutaneous wound repair process regarding their effects on migration and regeneration of basal keratinocyte5678. These galectins can be secreted by keratinocytes that are either proliferative, quiescent, or differentiable. Once secreted, the galectins can autocrinely or paracrinely modulate the early phases of wound healing by cross-linking the galectin ligands on various cells1. Although most of the epidermal stem cells with replicative potential exist within the basal layer—where both galectins are found in small amounts—the galectins secreted from the upper epidermal layers can paracrinely affect the basal epidermal stem cells, as well as the differentiable keratinocytes when a cutaneous wound has occurred910. Thus, galectin may have an important role in wound healing through not only promoting epidermal stem cell replication, but also accelerating keratinocyte migration. Through a combination of intrinsic and extrinsic aging, human skin undergoes several age-related epidermal changes, including increased time for keratinocyte migration and diminished replicative capacity of basal keratinocytes. These changes can potentially affect the process of wound healing. Therefore, we propose that a low level of galectin-3 and -7 expressions in the skin of aged subjects may likely be a contributing factor for delayed wound healing.
The present study also showed that the expression of galectin-3 and -7 is dependent on the differentiation status in normal keratinocytes. We also found a high level of galectin-3 expression in the dermis whereas galectin-7 expression was not observed. Interestingly, only adjuvant added SE model was stained with galectin-7. Because our group showed that these adjuvants are helpful for the reconstruction of SEs with good basal cell activity and epidermal epidermal differentiation3, these findings suggest that galectin-7 is a more sensitive marker for good basal keratinocyte activity and epidermal differentiation than galectin-3.
In conclusion, this study has demonstrated that galectin-3 and -7 in the epidermis are decreased in an age-dependent manner, possible association with impaired wound healing of the old. In addition, galectin-7 seems to be a more sensitive marker for differentiated and active keratinocytes.
ACKNOWLEDGMENT
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Korea (grant number: HN14C0094). This work was also supported by the Technology Innovation Program 10053020, Development of In Situ 3D Bioprinting System for Wound-Tailored Skin Regeneration funded By the Ministry of Trade, Industry & Energy (MI, Korea).
References
1. Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transl Med. 2014; 2:89. PMID: 25405164.
2. Bernerd F, Sarasin A, Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVBinduced sunburn keratinocytes. Proc Natl Acad Sci U S A. 1999; 96:11329–11334. PMID: 10500176.

3. Kim J, Jeong HS, Li H, Baek KJ, Kwon NS, Yun HY, et al. Effects of Cervi cornus Colla (deer antler glue) in the reconstruction of a skin equivalent model. Arch Dermatol Res. 2013; 305:85–89. PMID: 23011660.

4. Arqués O, Chicote I, Tenbaum S, Puig I, Palmer HG. Standardized relative quantification of immunofluorescence tissue staining. Protoc Exch. 2012; DOI: 10.1038/protex.2012.008.

5. Liu W, Hsu DK, Chen HY, Yang RY, Carraway KL 3rd, Isseroff RR, et al. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol. 2012; 132:2828–2837. PMID: 22785133.

6. Gendronneau G, Sidhu SS, Delacour D, Dang T, Calonne C, Houzelstein D, et al. Galectin-7 in the control of epidermal homeostasis after injury. Mol Biol Cell. 2008; 19:5541–5549. PMID: 18829868.

7. Chen HL, Chiang PC, Lo CH, Lo YH, Hsu DK, Chen HY, et al. Galectin-7 Regulates Keratinocyte Proliferation and Differentiation through JNK-miR-203-p63 Signaling. J Invest Dermatol. 2016; 136:182–191. PMID: 26763438.

8. Argüeso P, Mauris J, Uchino Y. Galectin-3 as a regulator of the epithelial junction: implications to wound repair and cancer. Tissue Barriers. 2015; 3:e1026505. PMID: 26451339.

9. Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006; 1760:616–635. PMID: 16478649.

Fig. 1
Expression of galectin-3 and -7 in human normal skin according to age and skin equivalents (SEs). Expression of galectin-3 and -7 was dependent on the differentiation status as well as age in normal keratinocytes. Galectin-7 is only expressed in the adjuvant added SE model.
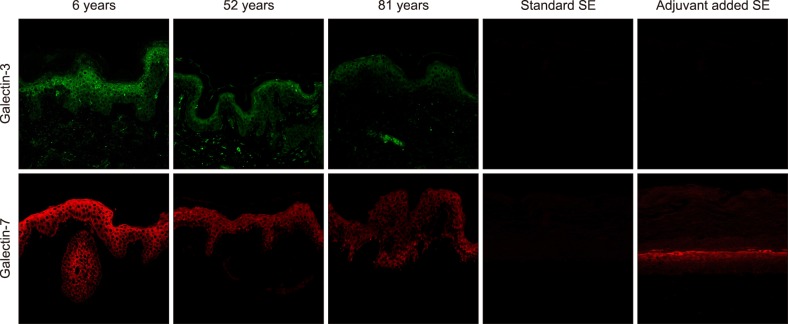
Table 1
The level of galectin expression per keratinocyte in human normal skin according to age and skin equivalents




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download