Abstract
Primary or metastatic malignant melanoma can mimic benign blue nevus in rare cases, making the diagnosis challenging. Herein, we report an exceptionally rare case of blue nevus-like melanoma and its blue nevus-like metastasis which was detected by catheterized urine cytology. The patient presented with blue-colored papuloplaques on his temple which were diagnosed as blue nevus-like melanoma on punch biopsies. While he was admitted for administration of chemotherapy, hematuria was detected. Catheterized urine cytology revealed singly scattered oval to spindle-shaped pigmented cells with a moderate degree of variation in shape and size. Many of them had small nuclei with indiscernible to inconspicuous nucleoli while only a few cells showed nuclear enlargement and nuclear hyperchromasia, which could be diagnostic pitfalls. Most of the cells on the smear were positive for HMB45 immunostaining, which confirmed the diagnosis of metastatic blue nevus-like melanoma. To the best of our knowledge, the present case is the first report describing cytomorphologic findings of blue nevus-like metastasis of melanoma in the urine specimen.
Malignant melanoma (MM), either primary or metastatic can rarely mimics benign blue nevus (BN), some of which can cause diagnostic difficulties due to the absence of obvious cytologic atypia, typical of conventional MM123456.
Cytomorphologic features of melanoma cells of conventional type identified in the urine cytology specimen have been described in several reports. Usually, cytologic detection of melanoma cells of conventional melanoma is not problematic as they exhibit characteristic cytologic features including high nuclear to cytoplasmic ratio, macronucleoli and anisocytosis78. However, there have been no reports of blue nevus-like metastasis of urinary tract detected by urine cytology, which could be a diagnostic pitfall owing to their bland cytomorphology. Herein, we present an extremely rare case of blue nevus-like melanoma (BNLM) and its blue nevus like metastasis, the latter of which was identified in the catheterized urine cytology.
A 61-year-old man presented with blue-colored papuloplaques on his left temple that had been present for over 1 year. Over the previous 2 months, the lesions had grown rapidly, without any symptoms. Physical examination revealed a large ill-defined dark blue patch studded with pinhead to bean sized cobblestone surfaced papulonodules and satellite papules (Fig. 1). Punch biopsies were performed in the center and at the periphery of the plaque. Microscopic examination of the central specimen revealed compact sheet-like proliferation of heavily pigmented epithelioid and, occasionally, short spindle cells in the entire dermis and subcutaneous fat layer, without junctional activity. One focus of tumor necrosis was found. The tumor cells showed mild to moderate nuclear enlargement and inconspicuous nucleoli. The mitotic count was up to 4 per 10 high power fields with deep and mid-dermal mitotic cells. The Breslow thickness was 4.53 mm. In the periphery of the lesion, the overall pattern of tumor cell infiltrates was similar to that of the center. However, many of the tumor cells had small pyknotic nuclei and the lesser remainders showed mild to (rarely) moderate nuclear enlargement, vesicular nuclei, and small nucleoli. There were rare exceptions of overtly atypical cells showing hyperchromatic enlarged nuclei, and bizarre giant cells. Due to these atypical cells, albeit rare, the histologic findings of peripheral lesion are not compatible with those of other benign dermal melanocytic lesions, i.e., common or cellular blue nevus and Ota nevus. Furthermore, there was no necrosis or mitosis in the dermis (Fig. 2). The tumor cells of both lesions were positive to HMB45, Melan-A and S-100 protein immunohistochemical staining. MIB-1 labelling index was 22%. Considering the clinicohistopathological findings, we diagnosed both lesions as BNLM.
Following the punch biopsy, a fluorodeoxyglucose-positron emission tomography scan revealed multiple lymph node and bone metastases, but no remarkable findings in the urinary tract. The patient was admitted for chemotherapy with dacarbazine 407 mg/day (250 mg/m2 body surface area). During the admission, hematuria of unknown duration was detected. Catheterized urine cytology on SurePath liquid-based preparation was performed. This demonstrated many singly scattered, pigmented cells admixed with red blood cells and occasional necrotic cells. The cells were oval to spindle-shaped with blunt ends and showed a moderate degree of variation in shape and size. Although difficult to recognize nuclear details due to heavy melanin pigment in many of the cells, most cells had small nuclei; only a few cells had nuclei showing mild to moderate nuclear enlargement and nuclear hyperchromasia. Most of the cells on the smear were positive for HMB45 and Melan-A; hence, they were determined to be metastatic BN-like melanoma cells (Fig. 3). However, some histiocytes and urothelial cells were negative for both stains. Subsequent abdominopelvic computed tomography revealed possible liver, spleen, and right kidney metastases, and suggested potential metastases in the bladder dome and lungs. Owing to the patient's poor general condition, cystoscopy with biopsy was not performed. The patient died from pneumonia 3 months after the initial diagnosis.
Primary or metastatic MM can mimic benign BN in rare cases, making the diagnosis challenging123456. The former was known as so-called ‘malignant blue nevus (MBN)4.’ However, this term MBN can cause confusion not only as a nevus is, by definition, benign, but also as it can be applied to several different situation including 1) MM arising in association with a preexisting BN and 2) less likely melanoma with architectural or cytologic features resembling BN but arising de novo. Thus, it has been suggested that the former should be designated as “melanoma arising in association with BN” or “melanoma associated with BN,” and the latter as “blue nevus-like melanoma5.” Of these, the present case represents a BNLM. Diagnosing BNLM is difficult not only because it is a rare disease entity, but also because there is no definite histologic criterion for diagnosing BNLM, particularly in de novo cases mimicking BN as in our case.
Metastatic MM can also resemble BN12, although this is rare. Primary tumors of BN-like metastasis include conventional MM and BNLM. Even in a patient with multiple cutaneous metastatic nodules, some nodules were conventional type while others were BNLM1.
Histologically, BNLM mimicking BN constitutes pigmented dendritic and spindle-shaped to epithelioid cells in the dermis and subcutaneous fat tissue, sparing the epidermis. The known histologic features distinguishing BNLM from benign BN are overt nuclear atypia, infiltrative borders, necrosis, frequent mitosis, and epithelioid cell morphology. However, some cases do not have these features, and instead contain a small number of tumor cells with mild to moderate nuclear atypia1, as demonstrated in the histology of the peripheral lesion and in the urine cytology in our patient. Given their clinical and cytologic similarities to BN, either BNLM or BN-like metastasis can be a diagnostic pitfall.
The histologic differential diagnosis includes pigment-synthesizing or animal-type melanoma (PSM or ATM), epithelioid blue nevus (EBN), and pigmented epithelioid melanocytoma (PEM). In contrast to BNLM, ATM has tumor cells with large and irregular nuclei, prominent nucleoli and more abundant cytoplasm. In our case, particularly in the periphery of the skin lesion and in the urine cytology, many of the pigmented tumor cells bear a close resemblance to BN cells showing minimal to mild nuclear atypia, which is supportive of a diagnosis of BNLM rather than ATM. However, ATM sometimes shows considerable overlap with BNLM; distinguishing them may be impossible. PEM is a newly suggested term that encompasses tumors previously diagnosed as both EBN and ATM9. This term has been suggested since both EBN and ATM share characteristic histology and indolent biologic behavior. Recent studies have suggested that fluorescence in situ hybridization, cytogenetic analysis, and gene expression profiling can be used as ancillary studies in establishing the differential diagnosis21011.
Clinically, BNLM occurs most commonly in the head and neck. Recent studies demonstrated that there are no significant differences between survival rate and risk of lymph node or distant metastases of BNLM and those of conventional type of melanoma6.
Although rare, urinary cytologic detections of metastatic melanoma to the urinary tract have been reported78. Hematuria is the most common presenting symptom in either primary or metastatic melanoma of the bladder. All of the reported cases of metastatic melanoma detected by urine cytology were conventional type melanomas, showing large epithelioid cells, marked anisocytosis, and macronucleoli. However, in the present case diagnosed as BNLM, urine cytology exhibited singly scattered, pigmented ovoid to short spindle-shaped cells with small pyknotic nuclei and only a few mildly enlarged hyperchromatic nuclei. The diagnosis of BN-like metastasis was suggested based on the cytologic features similar to the histology of the peripheral skin lesion, diffuse immunoreactivity to HMB45, and the result of imaging study, although not confirmed with a punch biopsy of the bladder. Due to the lack of obvious cytologic atypia, correlation with the clinical features was essential to make the correct diagnosis. To the best of our knowledge, this case is the first report of BN-like metastasis of melanoma to the urinary tract identified by urine cytology.
The urine cytological differential diagnosis includes melanin-containing histiocytes, such as in melanosis and melanuria, and benign BN8. Even though round-to-ovoid tumor cells closely resemble histiocytes in metastatic BN-like melanoma, the smear also includes short spindle cells with blunt ends or trophozoid cells, which differ from the cytomorphology of histiocytes. Another clue to the differential diagnosis is the moderate degree of variation in size and shape of the smeared cells. Furthermore, the diagnosis of metastatic BNLM in urine cytology can be confirmed by immunocytochemical staining for HMB45.
In this case, the tumor cells in urine cytology as well as in the peripheral skin biopsy included only a small portion of atypical cells. Therefore, to correctly diagnose BNLM or BN-like metastasis, it is important to recognize the morphologic features that can mimic BN cells. The diagnosis may be BN-like metastasis to the urinary tract when identifying BN-like cells in the urine cytology of a patient with a history of MM.
Figures and Tables
Fig. 1
A large ill-defined dark blue patch studded with pinhead to bean sized cobblestone surfaced papulonodules and satellite papules on the left temple.
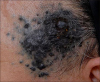
Fig. 2
Histologic figures of the specimen taken from the central (A, B) and peripheral (C, D) area of the lesion. (A) Solid proliferation of pigmented cells are noted in the entire dermis without junctional activity (H&E, ×40). (B) The tumor cells are composed of epithelioid and short spindle cells with mild to moderate nuclear enlargement, small nucleoli, and vesicular chromatin. A mitotic figure is identified in the deep dermis (arrowhead) (H&E, ×400). (C) The periphery of the lesion is composed of more spindle cells with minimal nuclear atypia (H&E, ×400). (D) Among the mildly atypical cells, there are rare bizarre atypical cells, visible on careful examination (arrow) (H&E, ×400).
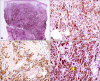
Fig. 3
Catheterized urine cytology on liquid-based preparation. (A) The specimen is moderately cellular and composed of oval to spindle-shaped pigmented cells showing moderate degree of variations in size and shape, admixed with many red blood cells (papanicolaou [PAP] stain, virtual slide). (B) Many of the pigmented tumor cells have small nuclei, some of which are obscured by melanin pigment (arrowheads) (PAP, ×1,000). (C) A small number of cells shows increased nuclear to cytoplasmic ratio (arrow) (PAP, ×1,000). (D) Another atypical cell exhibit an enlarged angulated hyperchromatic nucleus (arrow) (PAP, ×1,000). (E) The pigmented cells are positive on HMB45 immunostaining (arrowheads) (amino ethyl carbazol [AEC], ×400).

References
1. Busam KJ. Metastatic melanoma to the skin simulating blue nevus. Am J Surg Pathol. 1999; 23:276–282.

2. Campa M, Patel M, Aubert P, Hosler G, Witheiler D. Blue nevus-like metastasis of a cutaneous melanoma identified by fluorescence in situ hybridization. Am J Dermatopathol. 2016; 38:695–697.

3. Granter SR, McKee PH, Calonje E, Mihm MC Jr, Busam K. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus’. Am J Surg Pathol. 2001; 25:316–323.

4. Martin RC, Murali R, Scolyer RA, Fitzgerald P, Colman MH, Thompson JF. So-called “malignant blue nevus”: a clinicopathologic study of 23 patients. Cancer. 2009; 115:2949–2955.
5. Costa S, Byrne M, Pissaloux D, Haddad V, Paindavoine S, Thomas L, et al. Melanomas associated with blue nevi or mimicking cellular blue nevi: clinical, pathologic, and molecular study of 11 cases displaying a high frequency of gna11 mutations, bap1 expression loss, and a predilection for the scalp. Am J Surg Pathol. 2016; 40:368–377.
6. Loghavi S, Curry JL, Torres-Cabala CA, Ivan D, Patel KP, Mehrotra M, et al. Melanoma arising in association with blue nevus: a clinical and pathologic study of 24 cases and comprehensive review of the literature. Mod Pathol. 2014; 27:1468–1478.

7. Zogno C, Schiaffino E, Boeri R, Schmid C. Cytologic detection of metastatic malignant melanoma in urine. A report of three cases. Acta Cytol. 1997; 41:4 Suppl. 1332–1336.
8. Meunier R, Pareek G, Amin A. Metastasis of malignant melanoma to urinary bladder: a case report and review of the literature. Case Rep Pathol. 2015; DOI: 10.1155/2015/173870.

9. Zembowicz A, Carney JA, Mihm MC. Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol. 2004; 28:31–40.
10. Urso C, Ginanneschi C, Anichini C, Paglierani M, Saieva C, Pimpinelli N, et al. Animal-type melanoma: report of five cases with sentinel node biopsy and fluorescence in-situ hybridization analysis. Melanoma Res. 2014; 24:47–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download