Abstract
Extraskeletal osteosarcoma (ESOS) is a rare mesenchymal soft-tissue neoplasm that accounts for approximately 1% of all soft-tissue sarcomas. Over 70% of these malignant tumor progress to local recurrence and metastasis. It commonly metastasizes to the lungs, lymph nodes, bone, and skin and has a poor survival outcome. Cutaneous metastasis is exceedingly rare and known to be a sign of widespread metastases. We present a 57-year-old woman who presented with a rapidly growing protuberant mass on the scalp that was finally diagnosed as metastatic ESOS from a primary pancreatic ESOS. To our knowledge, there has been no reported case of pancreatic ESOS metastasizing to the scalp.
Extraskeletal osteosarcoma (ESOS) is an aggressive mesenchymal soft-tissue neoplasm accounting for 1% of all soft-tissue sarcomas and 2% to 5% osteosarcomas123. It can be diagnosed when the soft-tissue tumor appears with osteoid or cartilage matrix in a sarcomatous pattern but lacks any underlying bony connection4. Unlike osteosarcoma of the bone, which mainly occurs during the first two decades of life, this rare tumor occurs equally in both men and women in the fifth and sixth decades of life with a median age of 66 years24. Trauma and radiation therapy are well-known predisposing factors in the development of around 10% ESOS patients5. It clinically manifests as a painless lesion in the lower extremities, most commonly occurring in the muscles of the thigh followed by the retroperitoneum and upper limb167. The lung is the most common site for the metastasis of ESOS followed by the regional lymph nodes38. The reported skin metastases from ESOS have been exceedingly rare16. Because of its rarity, metastatic cutaneous ESOS has not been well characterized. Herein, we report a case of metastatic cutaneous ESOS on the scalp originating from a pancreatic ESOS.
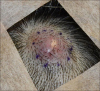
A 57-year-old woman presented with a rapidly growing protuberant mass on the scalp that she first noticed 1 month previously (Fig. 1). One year before, she had been admitted with a history of intermittent abdominal pain without fever, loss of weight, nausea, and other systemic symptoms. Work-up with computed tomography and magnetic resonance imaging (MRI) showed a solitary mass lesion in the tail of the pancreas. The pancreatic mass was diagnosed as a sarcomatoid carcinoma by fine-needle biopsy. She underwent a laparoscopic distal pancreatectomy with splenectomy. Intraoperative findings showed a pancreatic tail mass measuring 2.7×2.0×1.9 cm. Microscopic examination revealed atypical spindle-cell proliferation with focal osteoid formation with the background of a sarcomatous stroma (Fig. 2A, B). Immunohistochemistry showed positivity for CD99, vimentin and smooth muscle actin but was negative for cytokeratin, epithelial membrane antigen (EMA) and p63 (Fig. 2C, D). After surgery, she received adjuvant chemotherapy with FOLFIRINOX (folinic acid, fluorouracil, irinotecan, oxaliplatin) for twelve cycles and was referred to dermatology because of the newly arising scalp mass. Skin examination revealed a 1.8×2.0×0.5 cm, firm, erythematous nodule on the right parietal scalp. This nodule had increased in size over the previous month without any symptoms. Given the diagnosis of metastasis from the pancreas sarcomatoid carcinoma, an excisional biopsy was performed. The subsequent histopathological examination revealed a relatively well-demarcated subcutaneous tumor. The tumor was mainly composed of atypical spindle cells with mixed osteoid stroma. Tumor cells showed nuclear atypia and various pleomorphisms. Osteoclast-like multinucleated giant cells and hemorrhagic areas were also observed as well as multiple mitoses (Fig. 3A, B). On immunohistochemical examination, the mass was positive for CD99, vimentin, and p53 but was negative for cytokeratin, CD34, EMA, p63 and MDM-2 (Fig. 3C, D). The histopathological features were consistent with an ESOS. The final diagnosis was therefore retrospectively revised as pancreatic ESOS metastasizing to the scalp. Brain MRI was performed to check for any remaining tumors following biopsy. No abnormal lesion was observed within the excised scalp site. Given the diagnosis of metastatic pancreatic ESOS on the scalp, whole-brain radiotherapy is now planned in this case.
ESOSs are characterized by soft-tissue sarcomas that produce osteoid bone and/or cartilage but show no connection to the skeletal system189. There have been few reported cases of ESOS in the literature with only 300 cases reported since it was first described by Wilson in 194118. Although the lower extremities and retroperitoneum are common sites, ESOSs have been reported in other organs such as the larynx, esophagus, breast, liver, duodenum, kidney, urinary bladder, orbit, and skin145710. However, a pancreatic ESOS has not yet been reported in the literature. The present patient is therefore noteworthy as the first described case of a pancreatic ESOS that disseminated to the scalp. This rare case should be differentiated from other pancreatic tumors to establish the appropriate management and treatment plan. One could suggest a possible diagnosis for a pancreatic tumor of sarcomatoid carcinoma because of the similarity of the histopathology. In fact, this case was misdiagnosed as a pancreatic sarcomatoid carcinoma at initial assessment by the endoscopic ultrasound-guided fine-needle biopsy. A sarcomatoid carcinoma is a rare aggressive carcinoma with evidence of epithelial derivation and no specific line of mesenchymal differentiation. Seven cases have been reported with the average post-surgical survival of 6 months with its epithelial origin proved by the presence of epithelial immunomarkers11. However, immunohistochemical results of the pancreas and scalp from the present case were negative for cytokeratin without evidence of an epithelial component, thereby excluding sarcomatoid carcinoma and other metaplastic carcinosarcomas. In addition, the immunohistochemical result for p63, which has been proposed to be a helpful tool in diagnosing sarcomatoid carcinoma12, was negative, implying the final diagnosis as an ESOS rather than sarcomatoid carcinoma.
Histopathological features of ESOSs exhibit characteristic neoplastic osteoid, bony, and/or cartilage components with pleomorphic tumor cells. The variation is generally wide, ranging from a fibrosarcoma-like pattern to a pleomorphic undifferentiated sarcoma-like pattern. The tumor cells usually showed a spindle-cell proliferation with cytological atypia and mitotic activity. Osteoclastic multinucleated giant cells are frequently observed. There are three subtypes depending on the prominent components: osteoblastic, chondroblastic, fibroblastic subtypes1. The present case resembles osteoblastic ESOS in which the tumor cells are similar to malignant osteoblasts with relatively abundant osteoid material centrally located. ESOSs should be differentiated from other bone-forming tumors, including myositis ossificans, synovial sarcoma, extraskeletal chondrosarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, and other mesenchymal and epithelial neoplasms. Immunohistochemical analyzes would be helpful in these entities to make a differential diagnosis. Positivity for CD99, vimentin, osteonectin, and osteocalcin as well as negativity for cytokeratin, CD34, and S100 support the diagnosis of ESOSs7. CD99 is the most sensitive marker that is expressed in all types of osteosarcoma and MDM-2 can also be useful in distinguishing a low-grade ESOS from a benign lesion. Both the pancreatic and scalp specimens were negative for MDM-2, implying that this case is far less likely a case of low-grade ESOS.
ESOSs are usually aggressive. Over 70% patients experience local recurrence and metastasis. The overall 5-year survival is reported to range from 25% to 77% and patients with metastatic lesions have a morality rate over 60%37. In one case series, the median survival time was measured as 8 months for metastatic ESOSs with doxorubicin-based chemotherapy8. Metastases commonly occur in the lungs, bones, lymph nodes, brain, liver, and skin4. Metastasis to the skin is an exceedingly rare and known to be a sign of widespread metastases1. The scalp appears to be a common site for the cutaneous metastasis because of its hypervascularity causing frequent hematogenous spread13.
Because of the rarity of ESOS, there are no specific therapeutic guidelines for its treatment. There is even no agreement whether ESOSs could be treated in the same manner as osteosarcoma or a soft-tissue sarcoma. Although a wide margin for surgical resection is the preferred treatment option, chemotherapy and radiotherapy have also been attempted because of its poor survival outcome. Recently, a gemcitabin–docetaxel combination chemotherapy showed a partial long-term response without toxic complications8.
In conclusion, the current report described the first case of pancreatic ESOS that had spread to the scalp. Concerning the general poor prognosis of soft-tissue ESOS, clinicians and pathologists should be aware of this rare entity and keep in mind the necessity of a careful skin examination to ensure a correct diagnosis.
Figures and Tables
Fig. 2
(A) A tumor mass showing a solitary, sharply demarcated pancreatic mass (H&E, ×40). (B) Slightly spindled and epithelioid atypical tumor cells with pleomorphic nuclei and multiple mitoses (H&E, ×100). (C, D) Positive reactivity for high-molecular-weight (C) CD99 and (D) vimentin (C, D: ×200).
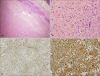
Fig. 3
(A) A subcutaneous tumor mass showing atypical spindle-cell proliferation (H&E, ×40). (B) Pleomorphic and hyperchromatic tumor cells with a prominent osteoid component and osteoclast-like giant cells (H&E, ×100). (C, D) Positive reactivity for high-molecular-weight (C) CD99 and (D) vimentin (C, D: ×200).

References
1. Lee WJ, Lee DW, Chang SE, Lee MW, Choi JH, Moon KC, et al. Cutaneous metastasis of extraskeletal osteosarcoma arising in the mediastinum. Am J Dermatopathol. 2008; 30:629–631.

2. Jour G, Wang L, Middha S, Zehir A, Chen W, Sadowska J, et al. The molecular landscape of extraskeletal osteosarcoma: a clinicopathological and molecular biomarker study. J Pathol Clin Res. 2015; 2:9–20.

3. Zreik RT, Meyer RG, Jenkins RB, Norambuena GA, Fritchie KJ. A rare pediatric example of subcutaneous extraskeletal osteosarcoma: a case report and review of the morphologic differential diagnosis. Am J Dermatopathol. 2016; 38:e44–e48.
4. Bots EM, Wismans PJ, Slobbe L. Pulmonary metastasised extraskeletal osteosarcoma. Thorax. 2016; 71:96.

5. Massi D, Franchi A, Leoncini G, Maio V, Dini M. Primary cutaneous osteosarcoma of the scalp: a case report and review of the literature. J Cutan Pathol. 2007; 34:61–64.

6. Covello SP, Humphreys TR, Lee JB. A case of extraskeletal osteosarcoma with metastasis to the skin. J Am Acad Dermatol. 2003; 49:124–127.

7. Narayanappa H, Khurian A. Primary duodenal extraskeletal osteosarcoma-a case report. J Histol Histopathol. 2014; 1.

8. Strippoli S, Traversa M, Cramarossa A, Popescu O, Lorusso V, Guida M. Long-term response of gemcitabine plus docetaxel chemotherapy regimen for extraskeletal osteosarcoma: a case report. Oncol Lett. 2015; 9:2567–2571.

9. Cevikol C, Gürer Eİ, Karaali K, Aydın AT, Lüleci E. Extraskeletal osteosarcoma arising in the pretibial subcutaneous tissue. Eur J Radiol Extra. 2004; 52:37–40.

10. de Maeyer VM, Kestelyn PA, Shah AD, Van Den Broecke CM, Denys HG, Decock CE. Extraskeletal osteosarcoma of the orbit: a clinicopathologic case report and review of literature. Indian J Ophthalmol. 2016; 64:687–689.

11. Kane JR, Laskin WB, Matkowskyj KA, Villa C, Yeldandi AV. Sarcomatoid (spindle cell) carcinoma of the pancreas: a case report and review of the literature. Oncol Lett. 2014; 7:245–249.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download