Abstract
Background
Skin cancer is the most common other primary cancer in patients with lymphoma. However, an intriguing association between cutaneous lymphoma and other primary cancers has been suggested in a few studies.
Objective
This study investigated other primary cancers in patients with cutaneous lymphoma to evaluate the risk for occurrence of each type of cancer.
Methods
We screened for other primary cancers in 428 patients with cutaneous lymphoma. Clinical features were analyzed according to the lineage and origin of the lymphomas. We calculated the standardized incidence ratio with statistical analysis for each group according to age.
Results
Among 330 patients with cutaneous T cell lymphoma and 98 with cutaneous B cell lymphoma, a total of 43 cancers in 38 patients were finally included. Other primary cancers were prevalent in patients with cutaneous B cell lymphoma and patients with secondary cutaneous lymphoma. However, those differences were not significant when the age was calibrated by multiple logistic regression. Metachronously higher standardized incidence ratios were observed for primary lung (standardized incidence ratio [SIR], 14.81; 95% confidence interval [CI], 3.05~39.54), skin (SIR, 68.05; 95% CI, 14.03~181.62), and breast (SIR, 12.91; 95% CI, 1.56~41.41) cancers with statistical significance.
Cutaneous lymphomas (CLs) are a diverse group of rare diseases characterized by cutaneous infiltration of malignant lymphocytic cells123. CL is generally divided into two subcategories: primary and secondary CL. Primary CLs (PCLs) are the second most common extranodal non-Hodgkin lymphomas. Secondary CLs (SCLs) are defined as systemic lymphoma accompanied by secondary spread to the skin134. Several epidemiological studies have demonstrated differences in the relative frequency and characteristics of CL in various regions, including the United States, Europe, and the Asia-Pacific region123. Likewise, other primary cancers in patients with CL may be different according to epidemiologic background. Several studies have evaluated the characteristics of other primary cancers in patients with primary cutaneous T cell lymphoma (PCTCL)456789 and primary cutaneous B cell lymphoma (PCBCL)10. However, an association between CL and other primary cancers has yet to be examined. Furthermore, there have not been any studies focused on Asian patients. The aim of the present study is to evaluate the relationship between CL and other primary cancers. We analyzed the entire history of other primary cancers in patients with CL and evaluated the standardized incidence ratio (SIR) for each type of cancer. There have not been any such extensive studies for all CLs comparing the relative frequency of other primary cancers in patients with PCL, SCL, cutaneous T cell lymphoma (CTCL), and cutaneous B cell lymphoma (CBCL). In this study, we included all PCL and SCL cases and all lineages of CL, comparing each group for their relative frequency of other primary cancers in Asian patients.
The present study was approved by the Institutional Review Board of the Asan Medical Center (IRB no. 2015-0263). A total of 428 patients with CL, of which 318 were diagnosed with PCL and 110 with SCL and confirmed by skin biopsy, were identified by searching the Asan Medical Center database between January 1997 and June 2016 (Table 1). Patients with pseudolymphoma and lymphomas of ambiguous phenotype were excluded. The medical records and clinicohistopathologic data were systematically reviewed and CL was classified according to the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) classification1. We defined PCL as showing skin-only disease at initial staging, irrespective of spread to other sites during follow-up. SCL was defined as having evidence of primary tumor in the lymph node or bone marrow that disseminated to the skin.
The following clinical data were collected from patient medical records: age, sex, site of lesion, duration of diagnosis, subtype of CL, types of other primary cancer, interval between onset of CL and other primary cancer, and survival. Histopathological diagnosis of primary cancer was made by excluding the possibility of metastasis from other cancers. By defining those cancers diagnosed more than 2 months after diagnosis of CL as metachronous primary cancers, the possibility of selection bias was minimized. Cancers diagnosed 2 or more months before the diagnosis of CL were considered prechronous cancers, whereas those cancers diagnosed within 2 months were synchronous primary cancers11.
All data were statistically analyzed by using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA). The p-values <0.05 were considered statistically significant. We used t-test and analysis of variance to investigate differences between group means. The chi-square, Fisher exact tests and multiple logistic regression were used to analyze differences between groups.
The relative risk of developing a metachronous primary cancer was evaluated by using SIRs1112. SIR is defined as the ratio of observed number of cancers divided by the expected number of cancers. The expected number of each cancer was generated by multiplying the person-years by the age- and sex-specific incidence rates for each cancer. The age- and sex-specific incidence rates of each cancer in general population were collected from the annual survey of National Cancer Information Center in year of 201413.
The statistical significance of the SIR was analyzed using the Poisson distribution and calculated 95% confidence intervals (CIs).
Among 428 patients with CL, there were 318 (74.3%) patients with PCL and 110 (25.7%) with SCL. The average age at diagnosis was 45.4 years (median age, 47 years). There were 240 male and 188 female patients, with a male-to-female ratio of 1.28:1. The number of patients with CTCL and CBCL were 330 (77.1%) and 98 (22.9%), respectively. The average age at diagnosis for the PCL and SCL groups was 43.9 and 50.5, respectively. The male-to-female ratio was 1.23:1 for the PCL group and 1.45:1 for the SCL group. The majority of patients (263/428, 61.4%) were diagnosed with PCTCL and 55 (12.9%) were diagnosed with PCBCL. Sixty-seven (15.7%) patients were diagnosed with secondary CTCL, whereas 43 (10.0%) patients were diagnosed with secondary CBCL (Table 1). Mycosis fungoides was the most common subtype, accounting for 93 patients (21.7%), followed by lymphomatoid papulosis (10.7%, n=46) and anaplastic large cell lymphoma (7.9%, n=34). Marginal zone B cell lymphoma was the most common subtype (5.6%, n=24) among patients with CBCL.
Among 428 patients with CL, 38 (8.9%) had one other primary systemic cancer and 5 patients had triple primary cancers; thus, a total of 43 patients had other primary cancers. Gastrointestinal cancers, including stomach and colorectal cancers, were the most common accompanying cancers (25.6%, 11/43), followed by lung cancer (14.0%, 6/43), skin cancer (11.6%, 5/43), and thyroid cancer (9.3%, 4/43), with other cancers accounting for the remaining 39.5% (17/43). Other primary cancers were more common in the SCL group (16.4%, 18/110) than in the PCL group (7.9%, 25/318) (p=0.011). The age of SCL group were significantly higher than PCL group (49.9±17.8 vs. 43.8±18.5, p=0.03). The incidence of other primary cancers in PCL or SCL group were not significantly different when the age was calibrated by multiple logistic regression (p=0.271). Urinary bladder cancer (n=2) and renal cell cancer (n=2) were observed in the PCL group, whereas prostate cancer (n=3) and thyroid cancer (n=3) were observed in the SCL group. According to the lineages of CL, other primary cancers were more prevalent in patients with CBCL (15.3%, 15/98) than in patients with CTCL (8.5%, 28/330) (p=0.049). The age of CBCL group were significantly higher than CTCL group (50.5±18.7 vs. 43.9±18.3, p=0.004). The incidence of other primary cancers in CTCL or CBCL group were not significantly different when the age was calibrated by multiple logistic regression (p=0.674). Lung (n=4), skin (n=4), and thyroid (n=3) cancers were more frequent in the CTCL group (Table 2, 3).
Of the 428 patients with CL, 30 (7.0%) cases of prechronous and synchronous primary cancers were observed. Gastrointestinal cancers (33.3%, 10/30) were the most common, followed by lung (10.0%, 3/30), prostate (10.0%, 3/30), thyroid (6.7%, 2/30), skin (6.7%, 2/30), brain (6.7%, 2/30), kidney (6.7%, 2/30), and urinary bladder (6.7%, 2/30) cancers, with other cancers accounting for the remaining 13.3% (4/30) (Table 3). Gastrointestinal cancers were common in patients with PCL (n=7), whereas prostate (n=3) and lung (n=2) cancers were common in patients with SCL. According to the histologic lineages, CTCL most frequently accompanied gastrointestinal cancers (n=6), followed by skin cancers (n=4) and thyroid cancers (n=3). CBCL most frequently accompanied gastrointestinal cancers (n=5), followed by prostate cancers (n=3) and lung cancers (n=2).
In the 428 patients with CL, 13 (3.0%) metachronous primary cancers were observed. Nine (2.1%) were in patients with CTCL and 4 (0.9%) in patients with CBCL. The average age at diagnosis of CL was 60.4 years (median age, 64 years). Lung (n=3) and skin (n=3) cancers were the most common metachronous primary cancers. All skin cancers were non-malanoma skin cancers including angiosarcoma (n=1), Kaposi sarcoma (n=1), basal cell carcinoma (n=1). The mean interval between diagnosis of CL and metachronous primary cancer was estimated as 33.7 months (median, 39 months). The average interval between diagnoses was 38.0±24.3 months in patients with CTCL and 24.0±21.9 months in patients with CBCL. When we analyzed metachronous primary cancers according to the site of lesions, lesions in the head and neck (n=5), trunk (n=4), upper extremities (n=2), and lower extremities (n=2) were observed. Considering the lineages of lymphomas, there was no significant difference in the frequency of metachronous primary cancers between patients with CTCL (2.7%, 9/330) and patients with CBCL (4.1%, 4/98) (p=0.493). However, the frequency of metachronous primary cancer between patients with PCL (1.9%, 6/318) and patients with SCL (6.4%, 7/110) were significantly different (p=0.018). A total of five patients with triple cancers were noted, of whom two had CTCL and three had CBCL, without statistical significance.
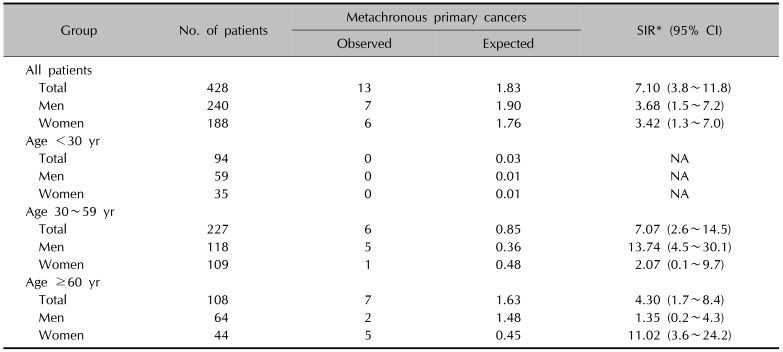
The SIR of each metachronous primary cancer was higher for the occurrence of lung (SIR, 14.81; 95% CI, 3.05~39.54), skin (SIR, 68.05; 95% CI, 14.03~181.62), and breast cancers (SIR, 12.91; 95% CI, 1.56~41.41), with statistical significance. The risks were also higher in the pancreas (SIR, 19.96; 95% CI, 0.51~93.34), thyroid (SIR, 7.70; 95% CI, 0.93~24.70), liver (SIR, 7.32; 95% CI, 0.19~34.23), and colon (SIR, 4.41; 95% CI, 0.11~20.60), without statistical significance (Table 4)12.
In this study, we retrospectively analyzed other primary cancers in Korean patients with CL. Although previous studies dealt with the specific lineages of CL, we surveyed all patients with CL and classified them for stratified analysis.
The risk of other primary cancers is generally increased in patients with CTCL4567891014. The percentages of other primary cancers in patients with CTCL estimated in several studies ranged from 5.5% to 11.2%814, with a relative risk of 1.04 to 2.459. In this study, 28 (8.5%) other primary cancers were observed in patients with CTCL. In a review of 672 patients with CTCL at the M.D. Anderson Cancer Center between 1979 and 1999, 112 (16.6%) had at least one additional cancer, with 37 (5.5%) that occurred after the diagnosis of CTCL, and the relative risk was estimated as a 1.79-fold risk8. In regards to the types of predominant metachronous cancers, non-Hodgkin lymphoma, Hodgkin lymphoma, lung714, colon7, biliary5, and vulvar8 cancers have been reported. Most recently, Amber et al.4 found 183 other primary cancers (6.3%) in a total of 2,923 cases of CTCL, with the SIR of 1.38 and, in their report, non-Hodgkin and Hodgkin lymphomas were the most predominant metachronous other primary cancers. In addition, the increased risks for bronchopulmonary cancers in female patients and urinary system cancers in patients with CD30+ cutaneous T cell lymphoproliferative diseases were newly noted4. We observed a total of 9 (2.7%) metachronous other primary cancers in 330 patients with CTCL. Considering the patients with CD30+ cutaneous T cell lymphoproliferative diseases, breast cancers (n=2), skin cancer (n=2; Kaposi sarcoma, angiosarcoma), and pancreatic cancer (n=1) were metachronously noted in a total of 91 patients, which is different from previously reported data. Unlike previous studies, we included non-melanoma skin cancers, suggesting an unreported or underestimated relationship between other primary non-melanoma skin cancers in CL.
In regard to CBCL, the only available cohort study was recently performed, and showed a high rate of other primary cancers (25.5%)10. However, we observed only 4 (4.1%) metachronous other primary cancers in 98 patients with CBCL. When including prechronous and synchronous other primary cancers, only 15 other primary cancers (15.3%) were detected, which is still lower than in the previous report. More studies are required to determine whether other primary cancers are more frequent in patients with CBCL.
The SCL group showed higher frequency for other primary cancers than the PCL group. The same tendency was observed when we focused on metachronous primary cancers, which also had a higher frequency in the SCL group than in the PCL group.
The overall incidences of other primary cancers in the present study in patients with CL were lower than those in previous studies4567891014. The reason for this relative lower risk might be derived from the ethnic difference and small sample size. Moreover, since this study was based on the single-centred study, it might cause the missing data and selection bias. Some of the previously reported literature did not clearly describe the sequence of diagnosis between CL and the other primary cancer. This point additionally might contribute to differences in stratified risk comparison. To demonstrate the more exquisite differences, further clinicoepidermiologic data are needed.
Two main hypotheses have been suggested to explain the vulnerability of patients with CL to other primary cancers. The first is the changes in functional T-cell subsets caused by a shift towards clonal T cells4. The relatively decreased number of interferon-γ-secreting T cells in patients with CTCL supports this hypothesis15. That deficiency in T cells of patients with Sézary syndrome is similar to that of patients with advanced acquired immunodeficiency syndrome (AIDS) also supports this hypothesis16. The second hypothesis is that chemotherapies for CL weaken the immune systems of patients with CL to other primary cancers4610. The incidence of other primary cancers in CTCL is highest within the first year of diagnosis4, supporting the role of immune dysfunction, rather than the influence of treatment for CL. One of the main objectives of this study was to explore the time interval between the diagnosis of CL and the diagnosis of other primary cancers to determine which hypothesis is more suitable to explain the risk of other primary cancers. Our data revealed a mean interval of 38.0 months in patients with CTCL, which is longer than previously reported4. Therefore, the influence of cytotoxic or chemotherapies in CL should not be ignored.
Although the overall incidence rate was measured lower than expected, the SIR for metachronous cancers is notable. The SIR was significantly higher in both sexes, irrespective of age (Table 4). In addition, the SIR of lung cancer, skin cancer and breast cancer were significantly higher. Recently, the deficiencies in the DNA repair pathways and chromosomal instability have been suggested as key factors in the development of CTCL17. This is supported by the fact that the expression of Ku70, which is involved in non-homologous end joining DNA repair, is significantly reduced in the T cells of patients with CTCL1819. The significantly prolonged repair time of damaged DNA in the lymphocytes of patients with CTCL also supports this17. Deficiencies in DNA repair proteins such as Ku70/Ku80 have also been suggested as a causal factor in lung cancer20 and breast cancer1821, and even in non-melanoma skin cancers22, which showed significantly higher SIR in patients with CL in our study. Although the underlying mechanisms are not fully understood, deficiency of DNA repair could be conceivable as the central concept for multiple cancer occurrences in patients with CL. Further genetic studies, including polymorphisms of the DNA repair genes in CL and other cancers, may help to better understand the cause and effect relationship. In conclusion, to the best of our knowledge, our study is the first report of the incidence of other primary cancers in Korean patients with cutaneous lymphoma. This study bears some limitations, such as being a retrospective study and having a small number of cases, along with being a single centre- based study. More patients are needed to shed further light on the association between CL and other primary cancers. Overall, for patients with CL, careful follow-up examinations are truly needed to detect other cancers as soon as possible.
ACKNOWLEDGMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. Lee JH, Lee JH, Yoo DS, Kang H, Kim GM, Park HJ, et al. Characteristics of primary cutaneous lymphoma according to WHO-EORTC classification in Korea. Clin Exp Dermatol. 2013; 38:457–463. PMID: 23611304.

2. Jenni D, Karpova MB, Seifert B, Golling P, Cozzio A, Kempf W, et al. Primary cutaneous lymphoma: two-decade comparison in a population of 263 cases from a Swiss tertiary referral centre. Br J Dermatol. 2011; 164:1071–1077. PMID: 21083546.

3. Lee WJ, Won KH, Won CH, Chang SE, Choi JH, Moon KC, et al. Secondary cutaneous lymphoma: comparative clinical features and survival outcome analysis of 106 cases according to lymphoma cell lineage. Br J Dermatol. 2015; 173:134–145. PMID: 25556641.

4. Amber KT, Bloom R, Nouri K. Second primary malignancies in CTCL patients from 1992 to 2011: a SEER-based, population-based study evaluating time from CTCL diagnosis, age, sex, stage, and CD30+ subtype. Am J Clin Dermatol. 2016; 17:71–77. PMID: 26386881.

5. Huang KP, Weinstock MA, Clarke CA, McMillan A, Hoppe RT, Kim YH. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sezary syndrome: evidence from population-based and clinical cohorts. Arch Dermatol. 2007; 143:45–50. PMID: 17224541.

6. Scarisbrick JJ, Child FJ, Evans AV, Fraser-Andrews EA, Spittle M, Russell-Jones R. Secondary malignant neoplasms in 71 patients with Sézary syndrome. Arch Dermatol. 1999; 135:1381–1385. PMID: 10566838.

7. Kantor AF, Curtis RE, Vonderheid EC, van Scott EJ, Fraumeni JF Jr. Risk of second malignancy after cutaneous T-cell lymphoma. Cancer. 1989; 63:1612–1615. PMID: 2924268.

8. Brownell I, Etzel CJ, Yang DJ, Taylor SH, Duvic M. Increased malignancy risk in the cutaneous T-cell lymphoma patient population. Clin Lymphoma Myeloma. 2008; 8:100–105. PMID: 18501103.

9. Olsen EA, Delzell E, Jegasothy BV. Second malignancies in cutaneous T cell lymphoma. J Am Acad Dermatol. 1984; 10:197–204. PMID: 6609176.

10. Chan SA, Shah F, Chaganti S, Stevens A, Amel-Kashipaz R, Vydianath B, et al. Primary cutaneous B-cell lymphoma: systemic spread is rare while cutaneous relapses and secondary malignancies are frequent. Br J Dermatol. 2017; 177:287–289. PMID: 27641754.

11. Bae SH, Seon HJ, Choi YD, Shim HJ, Lee JB, Yun SJ. Other primary systemic cancers in patients with melanoma: analysis of balanced acral and nonacral melanomas. J Am Acad Dermatol. 2016; 74:333–340. PMID: 26584878.

12. Breslow NE, Day NE. Statistical methods in cancer research. IARC Workshop 25–27 May 1983. IARC Sci Publ. 1987; (82):1–406.
13. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305. PMID: 28279062.

14. Väkevä L, Pukkala E, Ranki A. Increased risk of secondary cancers in patients with primary cutaneous T cell lymphoma. J Invest Dermatol. 2000; 115:62–65. PMID: 10886509.

15. Lee BN, Duvic M, Tang CK, Bueso-Ramos C, Estrov Z, Reuben JM. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin Diagn Lab Immunol. 1999; 6:79–84. PMID: 9874668.

16. Rook AH, Kubin M, Cassin M, Vonderheid EC, Vowels BR, Wolfe JT, et al. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol. 1995; 154:1491–1498. PMID: 7822812.
17. Thestrup-Pedersen K. Cutaneous T-cell lymphoma. A hypothesis on disease pathophysiology involving deficiency in DNA repair. J Eur Acad Dermatol Venereol. 2016; 30:1682–1685. PMID: 27501224.

18. Alshareeda AT, Negm OH, Albarakati N, Green AR, Nolan C, Sultana R, et al. Clinicopathological significance of KU70/KU80, a key DNA damage repair protein in breast cancer. Breast Cancer Res Treat. 2013; 139:301–310. PMID: 23624778.

19. Ferenczi K, Ohtola J, Aubert P, Kessler M, Sugiyama H, Somani AK, et al. Malignant T cells in cutaneous T-cell lymphoma lesions contain decreased levels of the antiapoptotic protein Ku70. Br J Dermatol. 2010; 163:564–571. PMID: 20408834.

20. Tseng RC, Hsieh FJ, Shih CM, Hsu HS, Chen CY, Wang YC. Lung cancer susceptibility and prognosis associated with polymorphisms in the nonhomologous end-joining pathway genes: a multiple genotype-phenotype study. Cancer. 2009; 115:2939–2948. PMID: 19408343.
21. Kumar A, Purohit S, Sharma NK. Aberrant DNA double-strand break repair threads in breast carcinoma: orchestrating genomic insult survival. J Cancer Prev. 2016; 21:227–234. PMID: 28053956.

22. Feehan RP, Shantz LM. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem J. 2016; 473:2973–2994. PMID: 27679857.

Table 1
Demographics of patients with cutaneous lymphoma
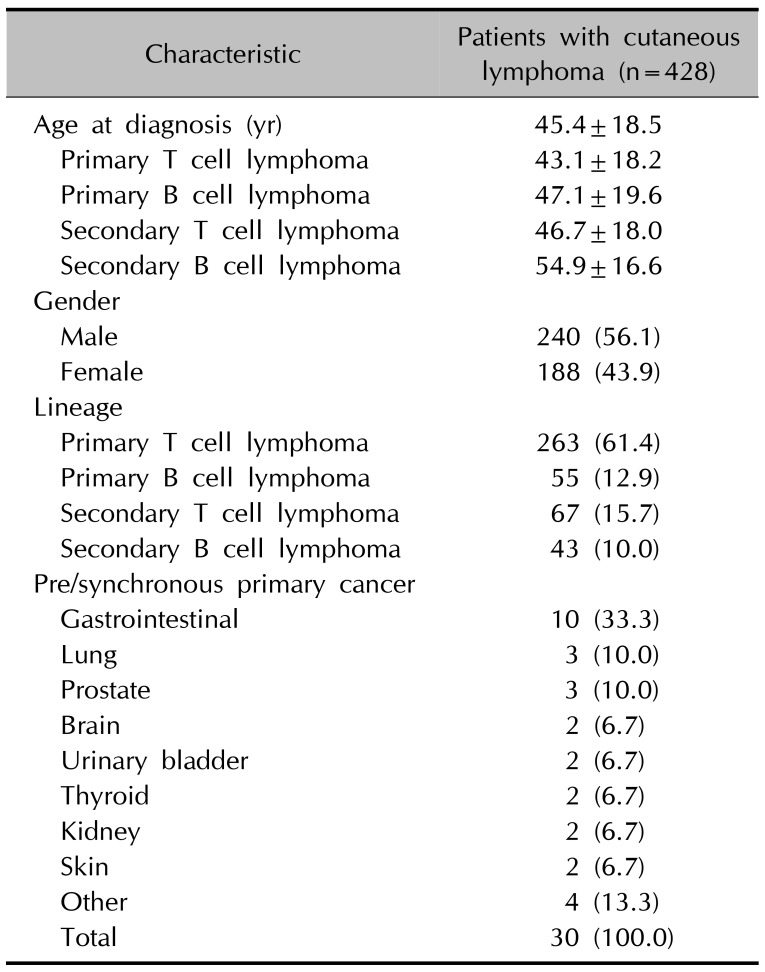
Table 2
Differential characteristics between patients with cutaneous T and B cell lymphoma
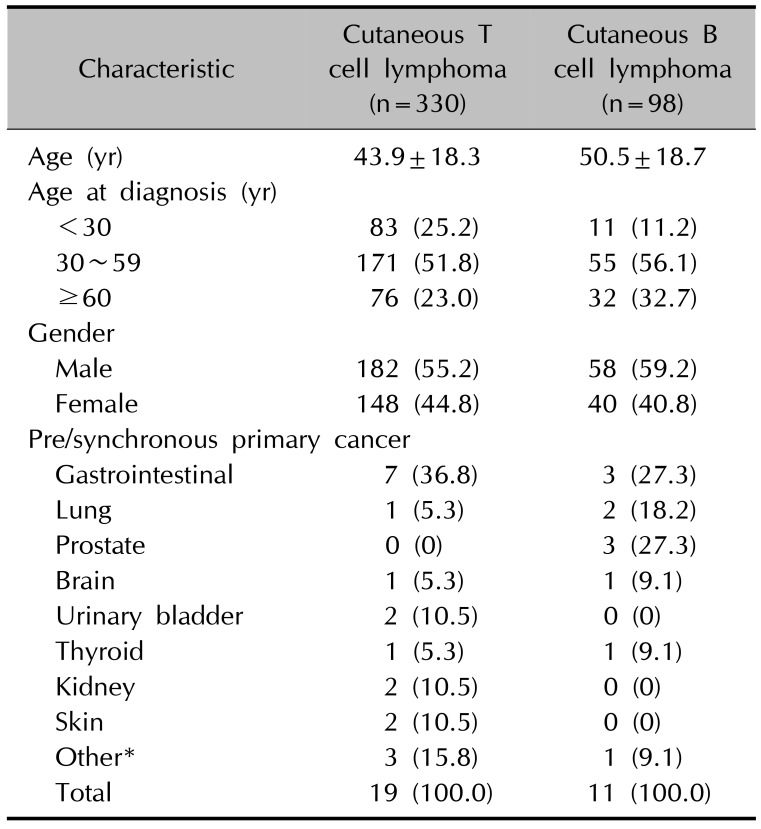
Table 3
Site of other primary cancers in patients with cutaneous lymphoma
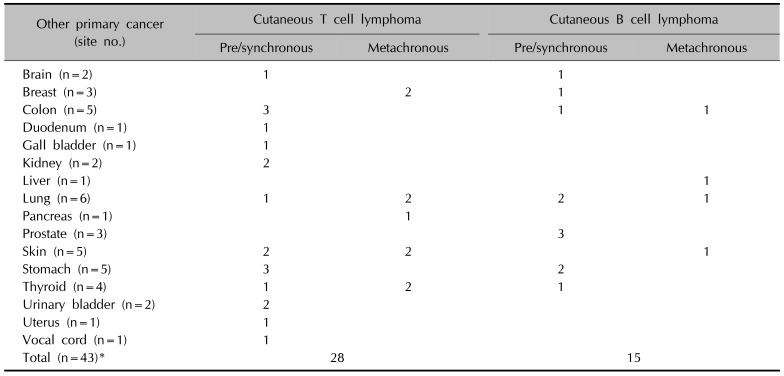
Table 4
A comparison of expected versus observed incidences of metachronous other primary cancers with different ages and gender
SIR: standardized incidence ratio, CI: confidence interval, NA: not assessed. *Let tk represent the person-time, Dk represent the observed events, Rk* represent the standard rate for the kth cell, where k=1, 2, …, M. Given this notation, the SIR is defined as SIR=
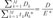
where the total number of events observed is 
and the total number of expected events is




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download