Abstract
Epidermal barrier formation and the maintenance of barrier homeostasis are essential to protect us from the external environments and organisms. Moreover, impaired keratinocytes differentiation and dysfunctional skin barrier can be the primary causes or aggravating factors for many inflammatory skin diseases including atopic dermatitis and psoriasis. Therefore, understanding the regulation mechanisms of keratinocytes differentiation and skin barrier homeostasis is important to understand many skin diseases and establish an effective treatment strategy. Calcium ions (Ca2+) and their concentration gradient in the epidermis are essential in regulating many skin functions, including keratinocyte differentiation, skin barrier formation, and permeability barrier homeostasis. Recent studies have suggested that the intracellular Ca2+ stores such as the endoplasmic reticulum (ER) are the major components that form the epidermal calcium gradient and the ER calcium homeostasis is crucial for regulating keratinocytes differentiation, intercellular junction formation, antimicrobial barrier, and permeability barrier homeostasis. Thus, both Ca2+ release from intracellular stores, such as the ER and Ca2+ influx mechanisms are important in skin barrier. In addition, growing evidences identified the functional existence and the role of many types of calcium channels which mediate calcium flux in keratinocytes. In this review, the origin of epidermal calcium gradient and their role in the formation and regulation of skin barrier are focused. We also focus on the role of ER calcium homeostasis in skin barrier. Furthermore, the distribution and role of epidermal calcium channels, including transient receptor potential channels, store-operated calcium entry channel Orai1, and voltage-gated calcium channels in skin barrier are discussed.
Calcium ions (Ca2+) serve as the universal signal to modulate various aspects of cellular functions in keratinocytes. The distribution and dynamic of Ca2+ in skin play an important role in epidermal homeostasis. In mammalian epidermis, a characteristic calcium gradient exists between lower and upper layers of epidermis, with low levels in basal and spinous layers and progressively increasing levels towards the stratum granulosum, and declining again in the stratum corneum (SC)123. The gradient of calcium across the epidermis plays a crucial role in the processes of keratinocytes differentiation and formation of the epidermal permeability barrier and allows the dynamic changes of calcium ions to generate calcium signaling45678. Recent evidences suggest that both Ca2+ release from intracellular stores and Ca2+ influx from extracellular sources are important for the regulation of epidermal structures and functions9101112. In this review, we focused on the origin and formation mechanism of epidermal calcium gradient and its roles in epidermal barrier homeostasis, keratinocytes differentiation, wound healing, and epidermal hyaluronan metabolism. We also discuss the homeostasis of Ca2+ in endoplasmic reticulum (ER) and ER stress response in keratinocytes and their implications in keratinocytes differentiation, permeability and antimicrobial barrier homeostasis, and cell-to-cell adhesion. The calcium-sensing receptor, transient receptor potential (TRP) channels, and Orai1 channel are also highlighted as the major constituents of calcium sensing and calcium influx in the keratinocytes.
Calcium gradients, formed by the relative concentrations of calcium ions in the extracellular and intracellular spaces, form the basis of skin function. Epidermal calcium gradients are generated by several mechanisms. Based on the Ca2+ gradient dynamics following acute SC barrier disruption (rapid disappearance and reappearance after 6 hours in parallel with barrier repair) and the evidence that reappearance of Ca2+ gradient following barrier disruption was accelerated by artificial barrier restoration, whereas delayed by inhibition of barrier recovery, Elias et al.3 suggested low rate of sustained transepidermal water loss with restriction of ion movement by an intact epidermal SC barrier accounts for the formation of the epidermal Ca2+ gradient. They also considered that these passive processes are sufficient to generate the Ca2+ gradient. A recent study suggested that epidermal tight junctions (TJs) also contribute to the generation/maintenance of the epidermal Ca2+ gradient. In addition to the SC barrier, TJs in the stratum granulosum function as a secondary barrier in skin by restricting the movement of ion, macromolecule, and pathogenic microbes13. Kurasawa et al.14 demonstrated that epidermal TJs contribute to Ca2+ gradient formation and epidermal differentiation in reconstructed human epidermis. These findings suggest that both the SC permeability barrier and the TJs are crucial to generate and maintain the epidermal calcium gradient by preventing Ca2+ diffusion from the stratum granulosum to the SC. Recent advances in calcium imaging techniques have advanced understanding of the main calcium sources required for the formation of the epidermal calcium gradient. Early investigations which employed Ca2+ capture cytochemistry and proton-induced X-ray emission method have suggested that the extracellular calcium content is critical cellular compartment for the epidermal calcium gradient115. However, these previous calcium measurement methods measure total Ca2+, not free Ca2+, and they require dehydration or fixation of tissues for measurement. To overcome these limitations, Celli et al.10 employed a fast fluorescence lifetime imaging system which measures ionic concentration from the decay of the ion-sensitive dye lifetime rather than its intensity which enables to visualize and quantify the spatial distribution of calcium in unfixed ex vivo epidermis and demonstrated that the majority of the Ca2+ in the stratum granulosm is found in intracellular stores such as the Golgi and the ER rather than in extracellular spaces. These findings suggest that ER calcium stores contribute to the epidermal calcium gradient. Calcium gradient and calcium signaling is crucial for the regulation of many skin functions. Below, we review the role of epidermal calcium ion and its gradients in permeability barrier homeostasis, keratinocytes differentiation and proliferation, cell-to cell adhesion, wound healing, and hyaluronan metabolisms in the skin.
The skin barrier function resides in the epidermis, particularly in the SC, the outermost cornified layer. SC is composed of corneocytes, which is the end product of keratinocytes terminal differentiation and SC intercellular lipid, the lamellar bilayers composed of ceramides, cholesterol, and free fatty acid. Lamellar bodies (LBs), the specialized organelles in the skin play a central role in the formation of the SC lipid, protein and anti-microbial barrier via delivering pro-barrier lipids and enzymes crucial for lipid processing along with proteases and anti-microbial peptides to the SC16. Prior studies demonstrated that the rate of LBs secretion, lipid synthesis and permeability barrier homeostasis are regulated by the changes in extracellular calcium concentration of the upper epidermis, which is triggered by permeability barrier disruption567. Acute barrier disruption by topical solvent application or tape-stripping induces an immediate depletion of both extracellular calcium ions in the epidermis, especially in the upper granular layers, and results in the loss of normal epidermal calcium gradient61718. The calcium levels in the upper epidermis then progressively restored over 6~24 hours in parallel with barrier recovery6717. Inhibition of extracellular calcium loss in the upper epidermis by immersion of barrier disrupted skin in the high calcium-containing solutions or occlusion with a vapor-impermeable membrane impairs the barrier recovery71920. These findings indicate that acute loss of calcium concentration in the stratum granulosm following barrier disruption is an important regulatory signal, initiating the immediate release of pre-stored LBs contents to the SC interstices, accelerated synthesis of new LBs and epidermal lipid synthesis leading to barrier repair. In addition to the extracellular calcium contents, calcium influx into keratinocytes also regulates barrier recovery. Lee et al.45 demonstrated that L-type Ca2+ channel blocker, verapamil reverses the high extracellular Ca2+-induced inhibition of barrier recovery. Both extracellular calcium and calcium influx via calcium channels regulates epidermal permeability barrier homeostasis. Later, Choi et al.21 demonstrated that high frequency sonophoresis or iontophoresis at energies that do not cause alterations in skin barrier function can trigger LBs secretion and cytokines expression to an extent comparable with barrier disruption via triggering the change in the epidermal calcium gradient. These observations suggest that alteration of calcium levels in the outer epidermis without influencing skin barrier function can be a strategy to enhance permeability barrier function.
Calcium ion is a major regulator in keratinocyte differentiation and proliferation82223. The skin is characterized by the vertical differentiation from basal layer to SC. The basal layer contains proliferating cells. As differentiation proceeds, keratinocytes progress upwards through the different epidermal layers along with the stage-specific changes in expression of numerous differentiation markers, and finally become the terminally differentiated corneocytes in the cornified layer of the SC. Calcium plays critical roles in the all processes of keratinocyte differentiation from the commitment to differentiation in the basal and spinous layer to the terminal differentiation in the stratum granulosum. Calcium regulates the transcription of all genes encoding keratinocyte differentiation- specific proteins. Activator protein-1 (AP-1) transcription factors are present in many keratinocyte-specific genes, including transglutaminase, loricrin, involucrin, profilaggrin, and other keratins to control the transcription of various differentiation markers24. Ng et al.25 revealed that gene encoding involucrin has AP-1 responsive element in the promoter region and the AP-1 site in the involucrin gene is essential for the calcium response, suggesting that nuclear Ca2+ regulates synthesis of differentiation specific proteins.
The calcium contents required for the stage-specific expression of differentiation-related proteins is different between each layers of the epidermis. For example, the extracellular calcium content required for expression of profilaggrin, the late differentiation marker, is higher than that required for the keratin 1 and keratin 10 expression8. These findings suggest that the epidermal calcium gradient is essential for proper epidermal differentiation and barrier formation. Calcium is also important in posttranslational processing of profilaggrin to filaggrin. Profilaggrin has N-terminal domain containing Ca2+-binding motifs, which share similarity with the EF-hands of the S100 Ca2+-binding protein family. Calcium, via binding to the head domain of profilaggrin, induces conformational changes, thereby exposing the crucial cleavage sites of profilaggrin to initiate the processing pathway26. During the terminal differentiation, many envelope precursor proteins, including involucrin, loricrin, elafin, small proline-rich proteins, filaggrin, and keratin are covalently cross-linked to form the cornified envelope by transglutaminase 1 and the transglutaminases mediate crosslinking of cornified envelope precursors in a calcium dependent-manner27. Therefore calcium modulates cornified envelope formation during terminal differentiation.
In addition to extracellular calcium ions, calcium sensitive proteins are also known to induce keratinocyte differentiation. Protein kinase C (PKC), which is activated by the rise in diacylglycerol and intracellular calcium induces differentiation markers of granular keratinocytes, including loricrin, filaggrin, and transglutaminases282930. Among the isozymes, PKCalpha and delta are activated by calcium ions in human and murine epidermis and regulate the extracellular calcium-induced transcription of these differentiated genes2930.
The mechanism of increased intracellular Ca2+ ([Ca2+]i) in the stratum granulosm layer in response to the elevation of extracellular calcium is now explained by the calcium-sensing receptor (CaSR) expressed in granular layer313233343536. CaSR, a subfamily of G protein-coupled receptors, in the plasma membrane senses the rise in extracellular calcium levels and activates phospholipase C, most likely through Gaq, which in turn generates inositol triphosphate (IP3), thereby releasing calcium from intracellular stores such as ER and Gogi and also stimulates Ca2+ influx via store-operated calcium channels343536. Previous studies suggested that the CaSR is involved in mediating calcium signaling during keratinocyte differentiation3132333435373839. Tu et al.40 evaluated the role of CaSR in vivo by generating keratinocyte-specific CaSR knockout mice and demonstrated that deletion of CaSR in keratinocytes causes the loss of epidermal calcium gradient, increased proliferation and a significant decrease in the amounts of the mid to late differentiation markers, and reductions in the number and the secretion of LBs at the stratum graulosum-SC interface, suggesting that CaSR is important for normal epidermal differentiation and barrier function in vivo.
Earlier studies have indicated that extracellular calcium is the major component forming the epidermal calcium gradient and critical for the signals of barrier repair in response to barrier perturbation1456715. However, recent studies from Celli et al.10 has demonstrated that the bulk of Ca2+ measured in the epidermis comes from intracellular Ca2+ stores such as the ER and the ER calcium depletion is an important signal for the terminal differentiation and epidermal barrier homeostasis. They found that external barrier perturbation by tape-stripping results in a marked loss of intracellular Ca2+ and activation of ER stress marker, XBP1 in murine skin to a comparable extent when treated with the sarco/endoplasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) inhibitor thapsigargin, which depletes ER calcium, indicating that most of the Ca2+ depletion from the stratum granulosum after barrier perturbation is derived from the intracellular ER calcium stores, triggering ER stress11. The same study also demonstrated that the ER stress induction in skin by chemical trigger, thapsigargin at the concentration that does not activate the apoptotic pathway but instead triggered physiologic unfolded protein response (UPR) stimulates the LBs secretion and the expression of caspase 14 and loricrin, mimicking the physiologic processes of barrier recovery independent of barrier disruption11. These results emphasize the important contributions of the intracellular calcium release, particularly from the ER in the regulation of keratinocyte differentiation and barrier homeostasis (Fig. 1). Release of ER calcium also has been demonstrated to be involved in the synthesis of antimicrobial peptide, such as cathelicidin and human beta-defensins via ER stress-induced ceramide metabolites (sphingosine-1-phosphate and ceramide-1-phosphate) pathways, indicating that various stresses to induce physiologic ER stress can enhance antimicrobial barrier function4142. However, when the ER stress is persistent or severe to exceed a given threshold, abnormal cell-to-cell adhesion, abnormal keratinization, or apoptosis can be triggered in keratinocytes43444546. Indeed, Savignac et al.45 demonstrated that the keratinocytes from patients with Darier's disease caused by mutations in the ER Ca2+ ATPase SERCA2 showed constitutive ER stress and increased sensitivity to ER stressors, which in turn, lead to abnormal cell-to-cell adhesion via impaired redistribution of desmoplakin, desmoglein 3, desmocollin 3, and E-cadherin. They also found that a pharmacological ER stress chaperone, Miglustat improved cell-to-cell adhesion in Darier's disease keratinocytes. Furthermore, the authors recently demonstrated that the effect of ER calcium release in keratinocytes by SERCA2 inhibitor thapsigargin on epidermal TJ barrier is different, depending on the degrees of ER Ca2+ depletion. Physiologic ER stress enhances TJ barrier, in contrast severe ER stress to induce abnormal UPR disrupts the structure and function of epidermal TJ partly via disorganization of perijunctional actin cytoskeleton. Taken together, ER Ca2+ homeostasis and ER stress are also crucial for the regulation of barrier formation, cell-to cell adhesion, antimicrobial barrier, and permeability barrier homeostasis.
Moreover, intracellular calcium dynamics play a role in keratinocyte migration and wound healing47. It is well known that asymmetries in the distribution of the intracellular Ca2+ concentration regulate cellular polarity, guidance, and migration via providing spatial and temporal information to control cellular extension and migration4849. Previous study has observed the asymmetric distributions of lamellipodial Ca2+ sparks in frequency during keratinocyte migration47. They also demonstrated that Ca2+-permeable channels within these cells are mechanically activated and among the mechanosensitive TRP channels, TRPV1 was revealed to be involved in the Ca2+ influx in response to the membrane tension during keratinocyte migration. These findings suggest that modulations of calcium and mechanosensitive TRP channels can be the potential strategies for wound healing, especially during the proliferation phase.
Recently, it is recognized that epidermal hyaluronan regulates epidermal differentiation and lipid synthesis/secretion, which in turn influence permeability barrier homeostasis through the interaction with its receptor, CD4450. Previously, our group demonstrated that permeability barrier perturbation induces the expression of hyaluronan and CD44 in murine epidermis and the epidermal calcium gradient change hyaluronan is the important signal for the barrier disruption-induced hyaluronan synthesis in keratinocytes51. In addition, the authors demonstrated that high frequency sonophoresis at the intensity that do not cause alterations in barrier function also induces epidermal hyaluronan and CD44 expression via triggering the change in the epidermal calcium gradient.
The effects of extracellular and intracellular Ca2+ on the keratinocyte differentiation and barrier homeostasis suggest that Ca2+ channels which mediate the Ca2+ influx into the cells exist in keratinocytes and regulate many functions in skin barrier homeostasis. Indeed, keratinocytes functionally express several types of calcium channels including TRP channels, components of the store-operated calcium entry (SOCE) pathway such as Ca2+ influx channel (Orai1) and endoplasmic Ca2+ depletion sensor (stromal interaction molecule 1 [STIM1]), and voltage-gated calcium channels (VGCCs) such as L-type calcium channel. The distribution of these channels are different in each layers of epidermis, therefore the response to calcium is distinct in different layers (Fig. 2).
TRP channels are widely expressed in the nervous systems and play an important role in processing sensory information such as itch and pain in response to a variety of environmental factors, such as temperature, physical or chemical stimuli525354. Growing evidences have indicated that most TRP channels are also expressed in keratinocytes and play an important role in the regulation of skin barrier homeostasis, keratinocytes differentiation/proliferation, and inflammation555657. TRP channels in keratinocytes also act as ‘cellular sensors’ that respond to changes in the environment, including temperature, mechanical, chemicals, osmolarity and pH and process those informations525354. Among the six subfamilies of TRP channels, we focus on the role of TRPV (vanilloid), TRPC (canonical), TRPA (ankyrin), and TRPM (melastatin) in skin barrier function.
Keratinocytes express five TRPV subfamilies including four nonselective cation channels (TRPV1, TRPV2, TRPV3, and TRPV4) and one highly Ca2+ selective channel (TRPV6)5859. Among the TRPV channels, TRPV1, TRPV3, and TRPV6 were demonstrated to regulate keratinocyte differentiation/proliferation. TRPV1 is activated by heat (>43℃), capsaicin and low pH and showed stronger expression in the stratum basale compared to upper layers of the skin606162. TRPV1 was demonstrated to be required for the endocannabinoid-mediated suppression of keratinocyte proliferation and induction of apoptosis63. Among TRP channels, TRPV3 is primarily expressed in the skin, especially in epidermal and follicular keratinocytes and activated by innocuous warm temperatures (>33℃), chemicals, and inflammatory mediators such as arachidonic acids64. The role of TRPV3 in keratinocyte differentiation, proliferation, and skin barrier function has been unraveled from TRPV3 knockout mice model and human disease caused by the mutations in TRPV3, the so-called “TRPV3 channelopathy”65666768697071. TRPV3-deficient mice show dry skin phenotype with defective barrier formation and altered late terminal differentiation along with abnormal hair morphogenesis68. Cheng et al.68 demonstrated that TRPV3 forms a signaling complex with transforming growth factor-alpha/epidermal growth factor receptor, two growth factors that regulate keratinocyte proliferation in basal layer and differentiation in suprabasal layers, to modulate the activity of transglutaminases to induce terminal differentiation and cornified envelope formation. In addition, transgenic mice with the gain-of-function mutation of the TRPV3 gene (TRPV3Gly573Ser) and patients with Olmsted syndrome (OMIM 607066) caused by the identical or other ‘gain-of-function’ mutation of TRPV3 showed similar clinical features characterized by a pruritic and hyperkeratotic skin inflammation with massive acanthosis and hyperkeratosis in histopathological examination697071. These findings indicate that hyperactive TRPV3 in keratinocytes disrupts the balance of keratinocyte proliferation and differentiation and emphasize its relevance in inflammation and pruritus. TRPV6, a highly Ca2+-selective channel, has been shown to be expressed in keratinocytes and play a crucial role in Ca2+/1,25-dihydroxyvitamin D3-induced differentiation of keratinocytes72. Knockdown of TRPV6 in human keratinocytes has been shown to impair the Ca2+-induced differentiated phenotype with inhibited expression of differentiation markers as involucrin, transglutaminase-1, and cytokeratin-10. 1,25-Dihydroxyvitamin D3 increases the expression of TRPV6 in human keratinocytes, which in turn mediates, at least in part, the pro-differentiating effects of 1,25-dihydroxyvitamin D3 by increasing Ca2+ entry, thereby promoting differentiation73.
Store-operated channels (SOC)-related Ca2+ entry is a Ca2+ entry pathway that is activated in response to depletion of Ca2+ stores within the ER, and contributes to the control of various cellular functions. Among the TRP channels, TRPC subfamily has been suggested to participate in SOC-related Ca2+ entry7475. TRPC1, TRPC4, and TRPC6 have been also implicated in the CaSR triggered elevation of [Ca2+]i and keratinocytes differentiation76777879. Knockdown of TRPC1 and TRPC4 in human keratinocytes has been shown to prevent the induction of Ca2+-induced differentiation76. It was also demonstrated that activation of TRPC6 with hyperforin induces full differentiation and inhibits proliferation similar to high [Ca2+]ex79. Previous studies demonstrated a defective SOC-related Ca2+ entry and reduced expression of TRPC1, TRPC4, and TRPC6 in psoriatic keraitnocytes. TRPC6 activation was observed to partly restore the disturbed differentiation and proliferation in psoriatic keratinocytes80. Furthermore, an up-regulation of TRPC1 was observed in keratinocytes of SERCa2+/− mice and Darier's disease patients and this upregulated TRPC1 was thought to augments cell proliferation and restrict apoptosis. These findings indicate an important role of TRPC channels-induced calcium influx in keratinocyte differentiation and proliferation81. However, it has also been demonstrated that ER Ca2+ release itself can promote keratinocyte differentiation, suggesting that keratinocyte differentiation is regulated by increased [Ca2+]i via both Ca2+ release from intracellular stores, such as the ER and Ca2+ influx mechanisms.
Denda et al.82 has demonstrated that several TRP channels such as TRPV1, TRPV4, and TRPA1 are involved in the regulation of epidermal permeability barrier homeostasis. They found that thermal (at 42℃) or pharmacological activation of TRPV1 (capsaicin) delayed barrier recovery, whereas thermal pharmacological activation of TRPV4 (4α-Phorbol 12,13-didecanone) accelerated barrier recovery, suggesting that TRPV1 and TRPV4 play important roles in skin permeability barrier homeostasis. A later study reported that a TRPV1 inhibitor compound, PAC-14028 improved epidermal barrier function in Dermatophagoides farina-and hapten-induced atopic dermatitis murine models83. TRPV4 that is activated by moderate heat (>30℃), hypo-osmolarity, and inflammatory metabolites has been demonstrated to regulate skin barrier formation. TRPV4-deficient mice showed the impaired epidermal barrier and it was demonstrated that TRPV4 is functionally co-expressed and interacts with β-catenin and E-cadherin, the crucial components linking adherens junctions and the actin cytoskeleton, thereby enhancing the formation of the epidermal TJ barrier848586. Further, pharmacological activation of TRPV4 has shown to strengthen the epidermal tightjunction barrier87. Other TRP channels, TRPA1 (below 17℃) and TRPM8 (below 22℃), which are expressed in keratinocytes and activated by low temperature have been demonstrated to play a role in epidermal barrier homeostasis. Denda et al.88 have demonstrated that brief exposure to cold (10℃ to 15℃) or pharmacological activation of TRPA1 (allyl isothiocyanate or cinnamaldehyde) accelerated barrier recovery. They later found that exposure to low temperature (<22℃) induced elevation of intracellular calcium in cultured human keratinocytes and topical application of TRPM8 agonists (menthol and WS 12) accelerated barrier recovery in vivo89. These findings indicate that modulation of TRP channels can be a therapeutic approach for skin diseases with barrier impairment such as atopic dermatitis and psoriasis.
Although many TRP channels in sensory nerve contribute to itch, TRPV3 and TRPV4 which are mainly expressed in keratinocytes, at much higher levels than those seen in neurons, have been suggested to be involved in non-histaminergic itch. The characteristic severe itch found in mice and humans with TRPV3 gain-of-function mutations strongly suggest the involvement of TRPV3 in keratinocytes in the production and transduction of itch signal possibly through the release of itch mediators to activate neurons in dorsal root ganglia. A number of candidate mediators such as prostaglandin E2 (PGE2), ATP, nerve growth factor, and thymic stromal lymphopoietin (TSLP) have been demonstrated to be released by TRPV3 activation on keratinocytes90919293. TRPV4 is an osmoreceptor in the skin and has been shown to be functionally required to generate dry skin–associated itch in mice. It was also found that TRPV4 mediate serotonin-evoked itch and 5-hydroxytryptamine signal is required for TRPV4- dependent chronic itch conditions94. These findings suggest a role of TRPV3 and TRPV4 in the link between keratinocyte calcium signal, epidermal barrier, and itch.
Gating of the Ca2+ release–activated Ca2+ (CRAC) channel is a classical instance of store-operated Ca2+ entry and recently Vandenberghe et al.95 identified that Orai1 is the main component of the store-operated current in human keratinocytes. Orai1 is activated by STIM1, the Ca2+ sensor of the ER. Upon ER calcium store depletion, STIM1 senses the ER Ca2+ reduction, followed by a local redistribution at sites of ER–plasma membrane apposition and subsequently recruits Orai1 to ER–plasma membrane contacts, where Ca2+ enters the cell through the opened Orai1 channels. Vandenberghe et al.95 also found that Orai1 is predominantly expressed in the basal layer of human epidermis and plays a critical role in the control of keratinocyte proliferation and polarized motility by enhancing focal adhesion turnover. Orai1 has shown to constitutively inhibit terminal keratinocyte differentiation. The keratinocytes from Orai1 knockout mice have also been shown as exhibit remarkably decreased migration, proliferation, and impaired differentiation, yielding impaired epidermis formation. Later, Darbellay et al.96 demonstrated that activation of Orai1 channel by SERCA inhibitor BHQ, which causes passive Ca2+ releases from the ER stimulates human keratinocyte proliferation and reverses corticosteroid-induced skin atrophy, suggesting that topical modulation of Orai1-mediated calcium influx can be a strategy to stimulate epidermal proliferation. The authors recently demonstrated that Orai1 is induced by ultraviolet B (UVB) in keratinocytes and plays a critical role in UVB-induced change such as epithelial proliferation, differentiation, barrier homeostasis and induction of TSLP and cyclooxygenase 2 in murine skin (not published yet).
Both Orai1 and TRPC channels are involved in the activation of store-operated Ca2+ entry in keratinocytes, however TRPC channels trigger keratinocytes differentiation, whereas Orai1/STIM1-mediated Ca2+ entry induces proliferation with suppressed terminal differentiation. The different effect of these two calcium channels-mediated SOCE on keratinocytes can be explained by the different distribution in epidermis. Orai1 is expressed mainly in the basal layer and TRPC channels are expressed more differentiated layers, thus the keratinocyte response to SOCE through these two channels might be different.
Furthermore, Wilson et al.97 demonstrated that Orai1 channel-mediated Ca2+ influx stimulate TSLP release from keratinocytes via the NFAT signaling and Orai1 channel is required for protease-activated receptor 2-evoked SOCE and TSLP secretion by keratinocytes, suggesting a role Orai1 in inflammation and itch in atopic dermatitis.
VGCC is categorized into the subtypes, L, P/Q, N, and R type. The L-type calcium channel, a high-voltage activated family of voltage-dependent calcium channel, is originally assumed to be expressed in excitable cells, but earlier studies have shown that the changes in skin surface electric potential regulate barrier homeostasis and the L-type channel blockers such as verapamil and nifedipine reversed the high extracellular calcium-induced delayed barrier recovery, suggesting the existence of L-type calcium channel in the epidermis498. Later, Denda et al.99 demonstrated the existence of functional L-type calcium channel in epidermal keratinocytes and their role in skin barrier homeostasis.
In conclusion, epidermal calcium gradient, ER calcium homeostasis, and calcium influx through TRP channels, Orai1, or VGCCs play a crucial role in keratinocyte differentiation, barrier formation, wound healing, and skin barrier homeostasis. In addition, keratinocytes by expressing numerous calcium channels can act as a biosensor that mediates, processes, or transmits the sensory signal in response to various physical or chemical stimuli. From the therapeutic perspective, it is of great importance to reveal the regulatory mechanisms and functions of calcium and related channels in skin barrier homeostasis.
Figures and Tables
 | Fig. 1Proposed role of endoplasmic reticulum (ER) calcium signaling in the permeability barrier homeostasis. Recent studies suggested that the intracellular calcium store such as the ER is the major compartment which forms the epidermal calcium gradient. Permeability barrier disruption stimulates Ca2+ release from ER in keratinocytes of stratum granulosum (SG), causing ER Ca2+ depletion and the loss of epidermal calcium gradient. ER Ca2+ depletion stimulates lamellar bodies (LBs) secretion and the expression of caspase 14 and loricrin, indicating that ER Ca2+ change is a critical signal for initiating the two key metabolic responses (lipid and protein barrier restoration) that lead to barrier recovery. The next physiological response to ER Ca2+ depletion is a rapid increase in store-operated Ca2+ entry, a mechanism involved in refilling of ER Ca2+ stores. Stromal interaction molecule 1 (STIM1) is an ER Ca2+ sensor that triggers the store-operated Ca2+ entry. In the SG, this store-operated Ca2+ entry is mediated by TRPC1 and TRPC4. This calcium influx through TRPC1 and TRPC4 further stimulates keratinocyte differentiation. SERCA: sarco/endoplasmic reticulum Ca2+-ATPase isoform. |
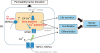 | Fig. 2The role of various calcium channels in skin barrier homeostasis and itch. Epidermal keratinocytes functionally express many calcium channels including transient receptor potential (TRP) channels and the components of the store-operated calcium entry (SOCE) pathway such as Ca2+ influx channel Orai1. TRPV6 expression is upregulated in the differentiated keratinocytes, where TRPV6 is implicated in the formation of epidermal calcium gradient and differentiation. TRPC channels in skin play a role in store-operated channels (SOC)-related Ca2+ entry pathways. TRPC1, TRPC4, and TRPC6 play a role in differentiation. In addition to TRPC channels, important player of SOC in skin is Orai1, which is mainly expressed in basal cell layer. Orai1 plays important roles in cellular proliferation, migration, and itch signals. TRPV3 and TRPV4 are activated by warm temperatures. TRPV3 plays a role in skin barrier formation and differentiation in keratinocyte through a transforming growth factor-alpha/epidermal growth factor receptor–complex, however, overactive TRPV3 stimulates abnormal keratinocytes proliferation. TRPV4 plays a role in the formation of epidermal tight junction (TJ). TRPV3 and TRPV4, which are strongly expressed in keratinocytes, have been implicated in itch, possibly by releasing various mediators to stimuli the sensory nerves. The heat-sensitive channel TRPV1 delays barrier recovery, in contrast the cold-sensitive channels TRPA1 and TRPM8 accelerate barrier recovery. TSLP: thymic stromal lymphopoietin. |
References
1. Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985; 84:508–512.

2. Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991; 127:57–63.

3. Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002; 119:1269–1274.

4. Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992; 89:530–538.

5. Lee SH, Elias PM, Feingold KR, Mauro T. A role for ions in barrier recovery after acute perturbation. J Invest Dermatol. 1994; 102:976–979.

6. Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992; 270:503–512.

7. Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992; 98:279–289.

8. Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989; 109:1207–1217.

9. Behne MJ, Sanchez S, Barry NP, Kirschner N, Meyer W, Mauro TM, et al. Major translocation of calcium upon epidermal barrier insult: imaging and quantification via FLIM/Fourier vector analysis. Arch Dermatol Res. 2011; 303:103–115.

10. Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T. The epidermal Ca(2+) gradient: measurement using the phasor representation of fluorescent lifetime imaging. Biophys J. 2010; 98:911–921.

11. Celli A, Mackenzie DS, Crumrine DS, Tu CL, Hupe M, Bikle DD, et al. Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br J Dermatol. 2011; 164:16–25.

12. Celli A, Crumrine D, Meyer JM, Mauro TM. Endoplasmic reticulum calcium regulates epidermal barrier response and desmosomal structure. J Invest Dermatol. 2016; 136:1840–1847.

13. Kirschner N, Rosenthal R, Furuse M, Moll I, Fromm M, Brandner JM. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013; 133:1161–1169.

14. Kurasawa M, Maeda T, Oba A, Yamamoto T, Sasaki H. Tight junction regulates epidermal calcium ion gradient and differentiation. Biochem Biophys Res Commun. 2011; 406:506–511.

15. Forslind B. Quantitative X-ray microanalysis of skin. Particle probe evaluation of the skin barrier function. Acta Derm Venereol Suppl (Stockh). 1987; 134:1–8.
16. Feingold KR. Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol. 2012; 132:1951–1953.

17. Mao-Qiang M, Mauro T, Bench G, Warren R, Elias PM, Feingold KR. Calcium and potassium inhibit barrier recovery after disruption, independent of the type of insult in hairless mice. Exp Dermatol. 1997; 6:36–40.

18. Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998; 111:1198–1201.

19. Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989; 30:323–333.

20. Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002; 119:1128–1136.

21. Choi EH, Kim MJ, Yeh BI, Ahn SK, Lee SH. Iontophoresis and sonophoresis stimulate epidermal cytokine expression at energies that do not provoke a barrier abnormality: lamellar body secretion and cytokine expression are linked to altered epidermal calcium levels. J Invest Dermatol. 2003; 121:1138–1144.

22. Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980; 19:245–254.

23. Pillai S, Bikle DD, Hincenbergs M, Elias PM. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988; 134:229–237.

24. Eckert RL, Crish JF, Banks EB, Welter JF. The epidermis: genes on-genes off. J Invest Dermatol. 1997; 109:501–509.
25. Ng DC, Shafaee S, Lee D, Bikle DD. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J Biol Chem. 2000; 275:24080–24088.

26. Presland RB, Bassuk JA, Kimball JR, Dale BA. Characterization of two distinct calcium-binding sites in the amino-terminus of human profilaggrin. J Invest Dermatol. 1995; 104:218–223.

27. Hitomi K. Transglutaminases in skin epidermis. Eur J Dermatol. 2005; 15:313–319.
28. Lee YS, Dlugosz AA, McKay R, Dean NM, Yuspa SH. Definition by specific antisense oligonucleotides of a role for protein kinase C alpha in expression of differentiation markers in normal and neoplastic mouse epidermal keratinocytes. Mol Carcinog. 1997; 18:44–53.

29. Denning MF, Dlugosz AA, Williams EK, Szallasi Z, Blumberg PM, Yuspa SH. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995; 6:149–157.
30. Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)alpha and PKCdelta. J Biol Chem. 2002; 277:17032–17040.

31. Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004; 35:265–273.

32. Tu CL, Bikle DD. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab. 2013; 27:415–427.

33. Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998; 273:23344–23352.

34. Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J Invest Dermatol. 2007; 127:1074–1083.

35. Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001; 276:41079–41085.

36. Blank JL, Ross AH, Exton JH. Purification and characterization of two G-proteins that activate the beta 1 isozyme of phosphoinositide-specific phospholipase C. Identification as members of the Gq class. J Biol Chem. 1991; 266:18206–18216.

37. Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J Clin Invest. 1996; 97:1085–1093.

38. Filvaroff E, Calautti E, Reiss M, Dotto GP. Functional evidence for an extracellular calcium receptor mechanism triggering tyrosine kinase activation associated with mouse keratinocyte differentiation. J Biol Chem. 1994; 269:21735–21740.

39. Tu CL, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J Invest Dermatol. 1999; 113:340–345.

40. Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012; 132:2350–2359.

41. Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A. 2016; 113:E1334–E1342.

42. Kim YI, Park K, Kim JY, Seo HS, Shin KO, Lee YM, et al. An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating β-defensin production. Mol Cell Biol. 2014; 34:4368–4378.

43. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89–102.

44. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.

45. Savignac M, Simon M, Edir A, Guibbal L, Hovnanian A. SERCA2 dysfunction in Darier disease causes endoplasmic reticulum stress and impaired cell-to-cell adhesion strength: rescue by Miglustat. J Invest Dermatol. 2014; 134:1961–1970.

46. Mauro T. Endoplasmic reticulum calcium, stress, and cell-to-cell adhesion. J Invest Dermatol. 2014; 134:1800–1801.

47. Graham DM, Huang L, Robinson KR, Messerli MA. Epidermal keratinocyte polarity and motility require Ca2+ influx through TRPV1. J Cell Sci. 2013; 126:4602–4613.

48. Tsai FC, Meyer T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol. 2012; 22:837–842.

49. Maroto R, Hamill OP. MscCa Regulation of tumor cell migration and metastasis. Curr Top Membr. 2007; 59:485–509.

50. Bourguignon LY, Ramez M, Gilad E, Singleton PA, Man MQ, Crumrine DA, et al. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol. 2006; 126:1356–1365.

51. Lee SE, Jun JE, Choi EH, Ahn SK, Lee SH. Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin Exp Dermatol. 2010; 35:650–657.

52. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001; 24:487–517.

53. Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002; 9:229–231.

54. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006; 68:619–647.

55. Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 2016; 9:E77.

56. Tóth BI, Oláh A, Szöllősi AG, Bíró T. TRP channels in the skin. Br J Pharmacol. 2014; 171:2568–2581.

57. Ho JC, Lee CH. TRP channels in skin: from physiological implications to clinical significances. Biophysics (Nagoya-shi). 2015; 11:17–24.

58. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007; 87:165–217.

59. Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011; 15:33–39.

60. Voets T. Quantifying and modeling the temperaturedependent gating of TRP channels. Rev Physiol Biochem Pharmacol. 2012; 162:91–119.

61. Radtke C, Sinis N, Sauter M, Jahn S, Kraushaar U, Guenther E, et al. TRPV channel expression in human skin and possible role in thermally induced cell death. J Burn Care Res. 2011; 32:150–159.

62. Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001; 285:1250–1252.

63. Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011; 131:1095–1104.

64. Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002; 296:2046–2049.

65. Steinhoff M, Bíró T. A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch. J Invest Dermatol. 2009; 129:531–535.

67. Nilius B, Bíró T, Owsianik G. TRPV3: time to decipher a poorly understood family member. J Physiol. 2014; 592:295–304.

68. Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010; 141:331–343.

69. Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009; 129:714–722.

70. Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012; 90:558–564.

71. Lai-Cheong JE, Sethuraman G, Ramam M, Stone K, Simpson MA, McGrath JA. Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol. 2012; 167:440–442.

72. Lehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, et al. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 2007; 282:22582–22591.
73. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007; 22:274–285.

74. Montell C. TRP channels: mediators of sensory signaling and roles in health and disease. Chem Senses. 2006; 31:A45–A45.
75. Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009; 106:3202–3206.

76. Beck B, Lehen'kyi V, Roudbaraki M, Flourakis M, Charveron M, Bordat P, et al. TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium. 2008; 43:492–505.

77. Fatherazi S, Presland RB, Belton CM, Goodwin P, Al-Qutub M, Trbic Z, et al. Evidence that TRPC4 supports the calcium selective I(CRAC)-like current in human gingival keratinocytes. Pflugers Arch. 2007; 453:879–889.

78. Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006; 452:43–52.

79. Müller M, Essin K, Hill K, Beschmann H, Rubant S, Schempp CM, et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J Biol Chem. 2008; 283:33942–33954.

80. Leuner K, Kraus M, Woelfle U, Beschmann H, Harteneck C, Boehncke WH, et al. Reduced TRPC channel expression in psoriatic keratinocytes is associated with impaired differentiation and enhanced proliferation. PLoS One. 2011; 6:e14716.

81. Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A, et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol Biol Cell. 2006; 17:4446–4458.

82. Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007; 127:654–659.

83. Yun JW, Seo JA, Jeong YS, Bae IH, Jang WH, Lee J, et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J Dermatol Sci. 2011; 62:8–15.

84. Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010; 285:18749–18758.

85. Sokabe T, Tominaga M. The TRPV4 cation channel: a molecule linking skin temperature and barrier function. Commun Integr Biol. 2010; 3:619–621.
86. Kida N, Sokabe T, Kashio M, Haruna K, Mizuno Y, Suga Y, et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch. 2012; 463:715–725.

87. Akazawa Y, Yuki T, Yoshida H, Sugiyama Y, Inoue S. Activation of TRPV4 strengthens the tight-junction barrier in human epidermal keratinocytes. Skin Pharmacol Physiol. 2013; 26:15–21.

88. Denda M, Tsutsumi M, Goto M, Ikeyama K, Denda S. Topical application of TRPA1 agonists and brief cold exposure accelerate skin permeability barrier recovery. J Invest Dermatol. 2010; 130:1942–1945.

89. Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol. 2010; 19:791–795.

90. Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009; 458:1093–1102.

91. Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006; 126:2664–2672.

92. Yamamoto-Kasai E, Yasui K, Shichijo M, Sakata T, Yoshioka T. Impact of TRPV3 on the development of allergic dermatitis as a dendritic cell modulator. Exp Dermatol. 2013; 22:820–824.

93. Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008; 28:13727–13737.

94. Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol. 2018; 141:608–619.

95. Vandenberghe M, Raphaël M, Lehen'kyi V, Gordienko D, Hastie R, Oddos T, et al. ORAI1 calcium channel orchestrates skin homeostasis. Proc Natl Acad Sci U S A. 2013; 110:E4839–E4848.

96. Darbellay B, Barnes L, Boehncke WH, Saurat JH, Kaya G. Reversal of murine epidermal atrophy by topical modulation of calcium signaling. J Invest Dermatol. 2014; 134:1599–1608.

97. Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013; 155:285–295.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download