Dear Editor:
Urticarial dermatitis (UD) represents a clinical subset of the dermal hypersensitivity reaction pattern1. Clinically, UD is characterized by urticarial and eczematous features, which vary in predominance over time. The urticarial component of UD differs from that of true urticaria, which resolves in <24 hours2. The differential diagnosis for a UD includes early bullous dermatosis, drug reaction, urticarial vasculitis, viral exanthema, and adult-onset atopic dermatitis (AD). Among them, adult-onset AD can manifest atypical clinical features34. Therefore, it may be difficult to distinguish UD and adult-onset AD. Furthermore, the precise histological distinction cannot always be made using routine histopathological techniques. In this study, we analyzed the histologic findings of UD to determine the status of the basement membrane (BM) in UD. We compared these findings to those of normal skin, and adult-onset AD. In doing so, we clarified the parameters that are useful for histopathological distinction between UD and adult-onset AD.
The study was approved by the institutional review board of Kyung Hee University Medical Center and was conducted according to principles of the Declaration of Helsinki (IRB no. KMC IRB 1504-03). Written informed consent was obtained from all participating patients. We included patients with UD and adult-onset AD who presented to the Department of Dermatology at Kyung Hee University Medical Center in Seoul, Korea between January 2010 and December 2014. Clinical evaluation of each patient included a review of the medical records. Punch biopsies of the lesional skin were performed. Hematoxylin-eosin (H&E) staining was used to determine general histopathologic changes. Normal skin was also collected from four volunteers. We used previously described clinical and histological to diagnose UD5. The AD diagnoses were made according to the Hanifin and Rajka criteria6. Adult-onset AD was defined as that beginning at age 20 years or older. The BM thickness was evaluated using periodic acid-Schiff (PAS) staining. The status of the BM was evaluated using immunohistochemical staining of type IV collagen and integrin α6.
Ten patients with UD (five males, five females; mean age=58.0±16.5 years; six samples from trunk, four from thigh) and six with adult-onset AD (three males, three females; mean age=44.3±15.3 years; five samples from the trunk, one from the thigh) were selected. Normal skin from four volunteers (two males, two females; mean age=33.8±15.8 years; three samples from the trunk, one from the thigh) was also collected. Fig. 1 displays the H&E staining, as well as clinical manifestations of the participants by group. Fig. 2 illustrates the differences in the staining intensities of PAS, type IV collagen, and integrin α6. There were no significant differences between the UD and normal groups with regard to the PAS-positive BM thickness (p=0.374). The BM thickness was significantly reduced in the AD group as compared to that in the normal and UD groups (p=0.01 and p<0.001, respectively). There were no significant differences between the UD and normal groups with regard to the staining intensities of type IV collagen (p=0.539) and integrin α6 (p=0.839) in the BM. Type IV collagen was significantly decreased in the BM of the AD group compared to that of the normal and UD groups (p=0.01 and p<0.001, respectively). Integrin α6 was significantly decreased in the BM of the AD group compared to those of the normal and UD groups (p=0.01 and p<0.001, respectively).
The histology of UD includes upper dermal perivascular lymphocytic infiltration with eosinophils and minimal associated epidermal spongiosis. This histology is identical to that which is thought occur in the dermal hypersensitivity reaction pattern, but is distinct from that seen in urticaria or solitary dermatitis. In the UD group in this study, there was a continuous linear band of BM with PAS staining. There were no significant differences in the UD and normal groups with regard to the type IV collagen and integrin α6 staining intensity in the BM. The clinical features of UD, including eczematous components and minimal epidermal spongiosis, can theoretically be detected histologically. However, we observed that the BM was intact in the UD group. This finding supports the hypothesis from Kossard et al.1, who suggested that UD represents the dermal form of an eczematous process, and is a subset of the dermal hypersensitivity reaction pattern.
Patients with adult-onset AD may have atypical morphology, localization, and distribution of AD compared to that of young patients with AD. Adult-onset AD, therefore, more commonly represents eczematous erythroderma34. Although gross spongiosis, lymphocytic exocytosis, and parakeratosis with irregular acanthosis may be microscopic clues indicating AD7, the precise histological distinction between adult-onset AD and UD is not always easily made. Nerve fibers often penetrate into the epidermis in skin with AD, which can cause pruritis8. Interleukin-13 also stimulates keratinocytes to generate matrix metal-loproteinase-9, which can degrade type IV collagen9. Therefore, the BM in skin with AD may become defective from these mechanisms. In the AD group, PAS staining revealed a thin linear band of BM with occasional faults and numerous strands extending into the connective tissue. There was significantly reduced type IV collagen and integrin α6 staining intensity in the BM of the AD group compared to those of the normal and UD groups. Similarly, Shin et al.10 compared the BM status in AD with those of chronic nummular eczema and normal skin. The group found that the BM is defective in AD, and has a low capacity to regenerate. Adult-onset AD and UD can sometimes be distinguished based on clinical clues, such as atopic history/background, facial involvement, and response to potent topical corticosteroids (all favoring a diagnosis of AD). In addition, however, special staining and evaluation of the BM status can also offer histologic clues in the differentiation of UD and adult-onset AD.
In conclusion, the BM is intact in UD, suggesting that its pathogenesis involves a dermal hypersensitivity reaction pattern. The UD and AD groups exhibited significant differences in the BM status. Therefore, histological examination using special staining may be useful in the differentiation of UD and adult-onset AD. Further, larger studies are needed to substantiate these findings.
Figures and Tables
Fig. 1
Clinical features and histological findings of urticarial dermatitis (UD) and adult-onset atopic dermatitis (AD). (A) Clinical features show urticarial erythematous papules, plaques, and patches on the right flank of a patient with UD. (B) Clinical features of a patient with adult-onset AD show erythematous excoriated papules on the abdomen. (C, F) H&E staining demonstrated mild papillomatosis and perivascular lymphocytic infiltration (C: ×100, F: ×400, respectively). (D, G) H&E staining showed mixed infiltration including lymphocytes, neutrophils, and eosinophils in the perivascular area (D: ×100, G: ×400, respectively). (E, H) H&E staining presented parakeratosis and acanthosis. Dermis shows mildly dilated vessels with perivascular lymphocytic infiltration (E: ×100, H: ×400, respectively).
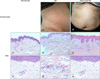
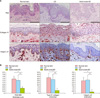
Fig. 2
Differences in basement membrane (BM) thickness and expression of type IV collagen and integrin α6 in normal skin, urticarial dermatitis (UD), and adult-onset atopic dermatitis (AD). (A) Periodic-acid-Schiff (PAS) staining demonstrated clear BM areas in normal skin. The UD group showed a continuous linear band of BM without discontinuity. The adult-onset AD group showed a thin linear band of BM with occasional faults and numerous strands extending into the connective tissue. Type IV collagen and integrin α6 were significantly decreased in the BM of the AD group compared with the normal and UD groups (×200; scale bar, 50 µm). (B) The mean of PAS-positive BM thickness in normal, UD, and AD groups was 1.37±0.26, 1.24±0.45, and 0.48±0.14 µm, respectively. The UD and normal groups did not show a significant difference in PAS-positive BM thickness (p=0.374). The AD group showed a significantly decreased thickness of BM compared with the normal group and UD group (p=0.01 and p<0.001, respectively). The UD and normal groups did not show significant difference in type IV collagen (p=0.539) or integrin α6 staining in the BM (p=0.839). The mean grade of staining intensity for type IV collagen in AD was 0.67±0.52, which is significantly lower than that observed in the normal (3.75±0.96) and UD (3.30±1.16) groups (p=0.01 and p<0.001, respectively). The mean grade of staining intensity for integrin α6 was 0.33±0.52 in AD, which is significantly lower than that in the normal (3.00±0.82) and UD (3.20±1.03) groups (p=0.01 and p<0.001, respectively). *p<0.05, **p<0.005.
ACKNOWLEDGMENT
This work was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1479).
References
1. Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol. 2006; 142:29–34.
2. Banan P, Butler G, Wu J. Retrospective chart review in a cohort of patients with urticarial dermatitis. Australas J Dermatol. 2014; 55:137–139.

3. Katsarou A, Armenaka M. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol. 2011; 25:12–18.

5. Hannon GR, Wetter DA, Gibson LE. Urticarial dermatitis: clinical features, diagnostic evaluation, and etiologic associations in a series of 146 patients at Mayo Clinic (2006-2012). J Am Acad Dermatol. 2014; 70:263–268.

6. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; Suppl 92. 44–47.
7. Choi YJ, Lee HJ, Lee DH, Woo SY, Lee KH, Yun ST, et al. Therapeutic effects and immunomodulation of suanbo mineral water therapy in a murine model of atopic dermatitis. Ann Dermatol. 2013; 25:462–470.

8. Kubanov AA, Katunina OR, Chikin VV. Expression of neuropeptides, neurotrophins, and neurotransmitters in the skin of patients with atopic dermatitis and psoriasis. Bull Exp Biol Med. 2015; 159:318–322.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download