Dear Editor:
The prevalence of skin cancer is increasing in Korea
1. Although increased public awareness has contributed to this trend, other risk factors or mechanisms in addition to ultraviolet-exposure may be involved. It is known that exposure to heavy metals contributes to the development of skin cancers
2. The main threats to human health are associated with toxic heavy metals, such as arsenic, cadmium, chromium, and lead
2. However, there have been few quantitative analyses of the effects of toxic heavy metal exposure on the risk of skin cancer. Therefore, this study examined exposure levels to toxic heavy metals in skin cancer patients and community controls through analysis of serum and urine samples.
A total of 138 biopsy-proven skin cancer patients (84 with basal cell carcinoma, BCC; 30 with squamous cell carcinoma, SCC; 14 with malignant melanoma; and 10 with other cancers, including dermatofibrosarcoma protuberans, eccrine porocarcinoma, and sebaceous carcinoma) and 142 community controls were included in the study. Controls volunteered for this study, and were selected based on the sex- and age-distribution ratio in the skin cancer group; controls underwent health examinations at the Dong-A University Hospital. The study was approved by the institutional review board of Dong-A University Medical Center (IRB no. 16-075). Written informed consent was obtained from all patients before participation.
Peripheral blood and urine samples were collected before breakfast. We used 10 mg/L multi-element calibration standards (Agilent Technologies, Santa Clara, CA, USA) to prepare 7 precalibration levels, from 0.05 ppb to 20 ppb. Then, a 1:100 dilution of the precalibrators was prepared in a basic diluent solution. Samples were prepared with a simple 1:10 dilution in this basic diluent. Rhodium (10 mg/L; Agilent Technologies) was used as an internal standard.
Heavy metal levels were measured using an inductively-coupled plasma-mass spectrometer (7700×; Agilent Technologies). To ensure accuracy, quality control was performed using standard samples (Whole Blood Metal Control Level 1, 2, Seronorm; SERO AS, Billingstad, Norway). Urinary creatinine level was measured, and the analytic values of urine samples were adjusted for creatinine.
All data were analyzed using SAS (ver. 9.4; SAS Institute, Cary, NC, USA). Gender and age compositions of both groups were confirmed by independent χ2 tests. The geometric mean (GM) and 95% confidence interval for each heavy metal concentration were presented as skewed distributions (skewness >0). An independent sample t-test was performed by logarithm transformation to compare the concentrations. The patient group was stratified according to skin cancer types and compared with the control. The p<0.05 was deemed statistically significant.
There was no significant difference in the demographic characteristics of the patient and control groups. Because of 3 specimen contaminations and 2 sampling failures, 133 blood samples (81 BCC, 28 SCC, 14 MM, 10 other) and 138 urinary samples from the skin cancer group were analyzed.
The patient and control groups did not show significant differences in serum arsenic, chromium, lead, or cadmium concentration (
Table 1). The GM of serum arsenic and cadmium levels in both groups showed no difference. Serum chromium and lead levels in the patient group tended to be higher, but did not reach statistical significance. In the analysis of heavy metal levels in the different types of skin cancer, serum lead concentrations in BCC patients were found to be significantly higher than those in the control group. The levels of arsenic, chromium, and cadmium were not significantly different among the various types of skin cancers.
Table 1
Geometric mean of serum heavy metal concentration
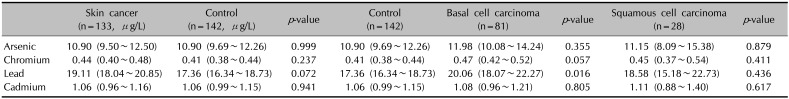
|
Skin cancer (n=133, µg/L) |
Control (n=142, µg/L) |
p-value |
Control (n=142) |
Basal cell carcinoma (n=81) |
p-value |
Squamous cell carcinoma (n=28) |
p-value |
|
Arsenic |
10.90 (9.50~12.50) |
10.90 (9.69~12.26) |
0.999 |
10.90 (9.69~12.26) |
11.98 (10.08~14.24) |
0.355 |
11.15 (8.09~15.38) |
0.879 |
|
Chromium |
0.44 (0.40~0.48) |
0.41 (0.38~0.44) |
0.237 |
0.41 (0.38~0.44) |
0.47 (0.42~0.52) |
0.057 |
0.45 (0.37~0.54) |
0.411 |
|
Lead |
19.11 (18.04~20.85) |
17.36 (16.34~18.73) |
0.072 |
17.36 (16.34~18.73) |
20.06 (18.07~22.27) |
0.016 |
18.58 (15.18~22.73) |
0.436 |
|
Cadmium |
1.06 (0.96~1.16) |
1.06 (0.99~1.15) |
0.941 |
1.06 (0.99~1.15) |
1.08 (0.96~1.21) |
0.805 |
1.11 (0.88~1.40) |
0.617 |

There were no significant differences between the patient and control groups in urinary arsenic, chromium, lead, or cadmium concentration. In the analysis of levels among different types of skin cancer, the concentrations were not significantly different (
Table 2).
Table 2
Geometric mean of urinary heavy metal concentration
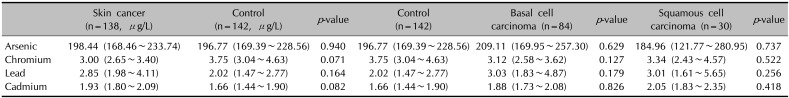
|
Skin cancer (n=138, µg/L) |
Control (n=142, µg/L) |
p-value |
Control (n=142) |
Basal cell carcinoma (n=84) |
p-value |
Squamous cell carcinoma (n=30) |
p-value |
|
Arsenic |
198.44 (168.46~233.74) |
196.77 (169.39~228.56) |
0.940 |
196.77 (169.39~228.56) |
209.11 (169.95~257.30) |
0.629 |
184.96 (121.77~280.95) |
0.737 |
|
Chromium |
3.00 (2.65~3.40) |
3.75 (3.04~4.63) |
0.071 |
3.75 (3.04~4.63) |
3.12 (2.58~3.62) |
0.127 |
3.34 (2.43~4.57) |
0.522 |
|
Lead |
2.85 (1.98~4.11) |
2.02 (1.47~2.77) |
0.164 |
2.02 (1.47~2.77) |
3.03 (1.83~4.87) |
0.179 |
3.01 (1.61~5.65) |
0.256 |
|
Cadmium |
1.93 (1.80~2.09) |
1.66 (1.44~1.90) |
0.082 |
1.66 (1.44~1.90) |
1.88 (1.73~2.08) |
0.826 |
2.05 (1.83~2.35) |
0.418 |

Data from animal and human studies have suggested that exposure to arsenic and chromium may be associated with an increased risk for various skin cancers
34. The International Agency for Research on Cancer classified cadmium as a human carcinogen (group 1)
5, based on sufficient evidence in both humans and animals, and lead as a possible human carcinogen (group 2B), on the basis of insufficient human data and sufficient animal data
6.
The geographical and environmental features of Korea, including numerous abandoned mines, increase the risk of soil and underground water contamination; exposure to air pollution is due to dense vehicle smoke and fine dust from factory districts in Korea and bordering countries
7. Moreover, a large proportion of the Korean diet includes fish, shellfish, seaweed, and kelp which often contain heavy metals. Therefore, an assessment of heavy metal exposure is required.
Interestingly, although arsenic is a well-known, heavy-metal skin carcinogen
48, our data showed no significant differences in exposure levels. Total arsenic includes various ions generated from all arsenic species. Generally, inorganic arsenic is considered more toxic than organic arsenic
89. Accordingly, the total arsenic level does not represent its toxicity or contribution to skin cancer. Alternatively, blood or urine arsenic levels may not represent past exposure, since arsenic is rapidly excreted in urine after causing cell damage
8. Therefore, detailed analysis of specific arsenic species concentrations should be performed to examine the contribution to skin cancer.
The serum lead concentration was significantly higher in the BCC group than in the control group, in spite of the non-significant difference in urine lead concentration. These data represent short-term exposure to lead in the BCC group. We examined smoking history and herbal ingestion, but there were no significant differences. Further study is required to clarify the contribution of lead to the development of BCC.
This study had a limitation, in that the cause of skin cancer or source of exposure could not be determined; only the current extent of heavy metal exposure was evaluated. However, this is the first study to investigate toxic heavy metal exposure in Korean skin cancer patients, in comparison with that in community controls.



