INTRODUCTION
Carbonated water, colloquially called sparkling water, is an aqueous solution of carbon dioxide.
1 Some of these have additives, such as sweeteners or fruit flavors, but plain carbonated water is composed mostly of water and carbon dioxide, with no other additives. For this reason, carbonated water is considered as a healthier beverage than conventional soft drinks and its consumption is increasing. According to a statistical survey on the production of food and food additives conducted by the Ministry of Food and Drug Safety in Korea,
2 carbonated water sales in 2014 saw a 100.63% increase from those in 2013, as compared to an 8.32% increase in conventional soft drink sales within the same period. Despite the increase in consumption, the general public is not well-informed of the negative effects of carbonated water on dental health.
Erosion of enamel is defined as the physical result of dental hard tissue being chemically etched away from the tooth surface by acid.
3 Previous studies have shown that dietary habits accompanied by excessive consumption of acidic foods and beverages increase enamel erosion.
3456 A healthy enamel surface is an important factor for a favorable prognosis during orthodontic treatment; since erosion of the enamel can occur due to increased consumption of acidic drinks, it can negatively affect the outcome of orthodontic treatment, especially on teeth that have been etched for orthodontic appliance bonding.
Not only conventional soft drinks, but also plain carbonated water is an acidic liquid. In previous studies, commercial carbonated waters showed a wide range of pH (pH 4.18-5.87)
78 and some showed a pH level that is below the critical level, pH 5.5, which is required for enamel demineralization. Since appliances that can carbonate water easily, such as soda carbonators, have become more common recently, carbonated water can easily be prepared and consumed at home. Consumers can manufacture carbonated water with varying degrees of carbonation, and a high degree of carbonation results in a high level of acidity, which can be harmful to teeth. Some oral health officials are aware of this problem and have suggested that the intake of carbonated water containing calcium ions is better than that of carbonated water without calcium ions.
Numerous studies
9101112 on the negative effects of conventional soft drinks on etched and sealed enamel have been conducted, and have shown that the drinks could cause erosion of etched enamel and dissolve adhesive material; however, studies that focus on the effects of plain carbonated water in this respect remain scarce. The purpose of this study was to determine the
in vitro influences of carbonated water on etched or sealed enamel in accordance with carbonation level and the presence of calcium ions.
MATERIALS AND METHODS
Specimen preparation and treatment
This
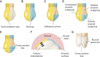
in vitro study was approved by the institutional review board at the Dental Hospital of Wonkwang University (No. WKDIRB201610-01). Seventy-five human premolars, extracted from young adults (age range, 20–29 years) for orthodontic purposes, were used in this study. Teeth with caries, erosion and/or other damage, or restorations were excluded. These teeth were obtained from various private practices and from clinics at the Wonkwang University Dental Hospital (Iksan, Korea). All teeth were cleansed and stored in normal saline solution. The roots of the teeth were cut off with a water-cooled, low-speed diamond saw for ease of use. The teeth were cleansed with nonfluoridated pumice and a rubber cup, thoroughly washed, and then air-dried. The left side of the buccal surface of each tooth was incisogingivally covered with melted acid-resistant wax (Sticky Wax; Kerr Co., Orange, CA, USA), and after cooling, the area was isolated from the test solution or artificial saliva during the experiment (
Figure 1A and 1D). This established the Unexposed enamel subgroups prior to immersion of the teeth in the test solutions, and allowed comparison of the exposed surfaces. The right side of the buccal surface, i.e., the unwaxed part of each tooth, was etched with 37% phosphoric acid (DB-Etching 37; Denbio, Gwangju, Korea) for 20 seconds, followed by cleansing for 20 seconds, and drying for 10 seconds (
Figure 1B). When the white, chalky surface appeared, Transbond XT Light Cure Adhesive Primer (3M Unitek, Monrovia, CA, USA) was applied on the cervical half of the etched surface, and this was then cured with an light emitting diode (LED) curing light (Ortholux; 3M Unitek) for 20 seconds (
Figure 1C). Thus, the occlusal part of the etched surface was designated as the Etched enamel subgroup of each tooth, and the cervical part of the etched surface was designated as the Sealed enamel subgroup of each tooth (
Figure 1D).
A soda carbonator with a 3-level LED fizz indicator (Source Sparkling Water Maker, SodaStream, NJ, USA), which is a popular commercial apparatus for manufacturing carbonated water, was used in this study. The LED fizz indicator shows the 3 stages of carbonation levels, by changes in LED lights when the carbonated level reaches a certain range. This was used to prepare carbonated water with different carbonation levels according to the manufacturer's instructions.
The tooth specimens were then randomly divided into 5 groups, in accordance with the carbonation level and the presence of calcium ions in the test solutions (i.e., a control group and 4 experimental groups):
Control group: Ultrapure deionized water
Group 1: Low-level carbonated water
Group 2: High-level carbonated water
Group 3: Low-level carbonated water with calcium ions
Group 4: High-level carbonated water with calcium ions
The product name, manufacturing method, and pH value of each test solution are listed in
Table 1.
Over a period of 7 days, all the specimens were submerged in each test solution for 15 minutes, 3 times a day, with 2-hour intervals. When the teeth were not submerged in test solutions, they were stored in artificial saliva at a constant temperature of 37℃. Before being submerged in the test solution, all the specimens were washed with ultrapure deionized water. The artificial saliva was prepared with 0.4 g NaCl, 1.21 g KCl, 0.78 g NaH
2PO
4·2H
2O, 0.005 g Na
2S·9H
2O, 1 g CO(NH
2)
2, and 1,000 mL of distilled and deionized water in the laboratory, as described previously.
4 Then, sodium hydroxide was added to this solution until the pH value was measured electrometrically as 6.75.
4 The test solutions and artificial saliva were remade and refreshed at the beginning of every experimental session.
After the immersion procedure, the specimens were washed with ultrapure deionized water and the wax was removed mechanically as a whole block without any effect on the enamel surface.
Measurement of microhardness
After the immersion procedure, 10 specimens from each group were cross-sectioned mesiodistally to divide the tooth into occlusal and cervical specimens. The cross-section was made at the level of the occlusal third (
Figure 1E). Each occlusal specimen was embedded in acrylic resin, such that the cut surface was exposed for the microhardness test (
Figure 1F). The surface of each specimen was ground to achieve a favorable flat enamel surface, using 600, 1,000, 1,200 grit silicon carbide abrasive paper sequentially, followed by polishing with 0.05-mm alumina slurry. All the specimens were kept in ultrapure deionized water prior to the microhardness test.
Microhardness tests on the Unexposed enamel subgroup and the Etched enamel subgroup were performed using a Vickers indentor attached to a microhardness tester (MXT-70 Microhardness tester; Matsuzawa, Tokyo, Japan). Microhardness tests were conducted under a 100-g load for 20 seconds dwell time. Three indentations per test were made on the outer enamel surfaces which were 10, 55, or 100 µm apart, making rows toward the dentin. Three tests were performed on each subgroup (
Figure 1F). Three rows of indentations were made with a distance of at least 300 µm. The length and width of each indentation were measured and the Vickers hardness number was calculated from the obtained values by the indentation apparatus of a measuring microscope. The microhardness was determined using the average value of 3 indentations per test and 3 rows were assessed for each subgroup.
Observation of specimen surface
After the immersion procedure, 5 specimens from each group were cross-sectioned incisogingivally to divide the tooth into buccal and palatal specimens (
Figure 1G). Each buccal specimen was kept in a desiccator containing CaCl
2 for 3 days, affixed to scanning electron microscopy (SEM) stubs, coated with platinum twice with a 108 Auto Sputter Coater (Cressington, Watford, UK), and was then examined with a JSM-6360 SEM (JEOL, Tokyo, Japan) at 15 kV, at different magnifications.
Statistical analysis
The microhardness of each Unexposed enamel subgroup was also compared (
Table 2). The Shapiro-Wilks test found a lack of normality of the distribution of the microhardness of each Unexposed enamel subgroup. Thus, the nonparametric Kruskal-Wallis test was used for analysis, and showed no significant difference (
p > 0.05).
Descriptive statistics were calculated for the microhardness of each subgroup. Statistical analyses were performed using the Predictive Analytics Software (version 18.0; SPSS Inc., Chicago, USA).
To assess microhardness changes of each group after the exposure to test solutions, microhardness of the Unexposed enamel subgroup and the Etched enamel subgroup were compared (
Table 2). Difference values between subgroups in the Control group and Groups 1, 3, and 4 were normally distributed. Paired
t-tests were used for the Control group, and Groups 1, 3, and 4, while the Wilcoxon signed rank test was used for Group 2. The nonparametric Kruskal-Wallis test and Mann-Whitney
U-test with Bonferroni correction were used as
post-hoc comparisons to compare difference values between subgroups (
Tables 3 and
4).
DISCUSSION
The objective of this study was to determine the
in-vitro effect of carbonated water on erosion of the etched enamel and degradation of the adhesive material in accordance with the carbonation level and the presence of calcium ions. This study revealed that carbonated water with different carbonation levels affects etched and sealed enamel to varying degrees. Higher carbonation levels show a higher tendency for enamel erosion, and addition of calcium alleviates this tendency (
Table 3).
In this study, the waxed part of the teeth was used as the Unexposed enamel subgroups for comparing conditions after etching and exposure to test solutions. Prior to the experiment, we conducted a pilot study, which showed that coating and mechanical removal of the wax had no effect on microhardness and the surface structure of the enamel.
We designed this study to reproduce the oral environment
in vivo, including the immersion time, protocol, and storage medium in intervals, based on previous studies.
1112 Since meal time was assumed to be 15 minutes, the teeth used in the experiment were submerged in test solutions for 15 minutes at a time and in artificial saliva between experimental sessions to simulate the oral environment. This procedure was repeated 3 times a day to simulate every meal.
This study had some limitations because of the
in-vitro study design. We considered that the post-eruptive age of teeth could affect enamel surface structures and microhardness.
13 Thus, the premolars extracted from young adults were used in this study. Nonetheless, exposure level to acidic beverages or food before extraction and its impact on the teeth could not be estimated. Furthermore, we used artificial saliva at a constant temperature of 37℃ to simulate the oral environment. To exclude the possibility of remineralization effects by calcium ions in saliva and to focus on the role of calcium ions in the test solutions, we used artificial saliva that did not include calcium ions.
5 Previous studies have shown that saliva has protective effects against demineralization of teeth, not only due to its buffering capacity, but also by enhancing ion-induced remineralization.
614 Thus, in the oral environment
in vivo, with normal saliva secretion and function, protective mechanisms against demineralization might have a stronger effect than the
in-vitro environment. Additionally, the effects of biofilms on the reactivity of the enamel in the oral environment could decrease demineralization by the carbonated water. The effects of carbonated water on the etched and sealed enamel must be further studied
in vivo to be verified in an oral environment.
To assess dental erosion, the microhardness test and SEM test were used. Microhardness tests directly assess the condition of a tooth surface and quantify dental erosion.
15 SEM tests, on the other hand, allow visual observation of the enamel surface change. These 2 test methods are complementary.
Microhardness of teeth showed significant differences and different features were observed in SEM images. The result from this study showed that the teeth exposed to carbonated water had more enamel erosion than those of the Control group. Other studies
41112 have analyzed the effect of acidic drinks, such as soft drinks on the teeth, but they have not mainly dealt with the effect of carbonated water. The microhardness values of teeth exposed to acidic drink were significantly lower than those of the control group.
16
Erosion of teeth exposed to carbonated water (Groups 1–4) as observed by SEM was greater than that observed in a previous study.
11 In a previous study, commercial sparkling mineral water was used, whereas carbonated water made by a soda carbonator was used in this study. In our pilot study, we found that carbonation levels vary by products similar to previous findings
817 and a soda carbonator can manufacture water with higher levels of carbonation. Accordingly, the carbonated waters used in this study were predicted to result in a higher tendency for enamel erosion than commercial sparkling waters.
The critical pH of demineralization, defined as the highest pH at which saliva crosses the saturation line, has been established as pH 5.5.
18 When exposed to an acidic solution with a lower pH than this critical pH, enamel dissolution is initiated. We used manufactured carbonated water of two different carbonation levels, and their pH levels were lower than the critical pH, even at the lowest carbonation level. Furthermore, in this study, the teeth were intermittently exposed to carbonated waters and artificial saliva during 7 days, and demineralization occurred even during this short period. Since the orthodontic treatment period is estimated to be about 1.5 years or more, carbonated water can cause greater harmful effects.
A greater amount of erosion was seen in the high-level carbonated water group than in the low-level carbonated water group. This tendency was not influenced by the presence of calcium ions. This demonstrated that higher levels of carbonation have a more destructive effect on enamel surface. In this study, higher levels of carbonation resulted in water with a more acidic pH value. However, this result might have been overestimated because the study design did not include a positive control. Nevertheless, it could provisionally be concluded, based on similar results for potential of erosion after consumption of acidic drinks that have previously been reported.
111619
In particular, the amount of erosion differed significantly between Group 1 and Group 3, whereas there was no significant difference between Group 2 and Group 4. These results reflect that calcium ions reduced erosion of the enamel, such that the microhardness was decreased in Group 3 compared to the Control group, and in Group 4 as compared to Group 1. The presence of calcium ions influenced the dissolution equilibria of the dental enamel. Nonetheless, addition of calcium ions seemed to have a limited effect in high-level carbonated water. This result corresponded with that of a previous study.
20 In terms of assessing the effect of the calcium ions in carbonated water, the calcium ion concentration was adjusted to 100 mg/L that resulted in an approximately 50% reduction in dissolution of hydroxyapatite in the previous study
20; this reflects the highest calcium ion concentration among commercial mineral waters. Additionally, if we used human saliva, the calcium ions influence of on reduction of enamel erosion could be decreased due to remineralization. Therefore, further studies with human saliva are needed to confirm the role of calcium ions in carbonated water.
In a previous study,
21 calcium ions were released into the carbonated water after bovine teeth were immersed in carbonated water. The concentration of calcium ions from the bovine teeth increased over time and continued, and moreover detection of the calcium ion was performed after several hours of immersion in carbonated water. In this study, the immersion time was shorter than that in the previous study,
21 and the test solutions were remade and refreshed at the beginning of every experimental session to minimize the effect of the calcium ions released from the teeth. In addition, calcium ions were added as calcium chloride, based on a previous study,
20 because sodium chloride has been reported to have a negligible influence on the potential of erosion.
A SEM test showed significant features associated with these erosive tendencies. Phosphoric acid etchant changed the enamel morphology of the Etched enamel subgroup in the Control group, and this showed a typical etching pattern, type 2, as a predominant pattern.
22 When the etched enamel surface was exposed to carbonated water, this well-defined pattern was collapsed by the destructive effect of the carbonated water. The level of influence varied according to the carbonation levels and the presence of calcium ions. These observations accorded with the results of the microhardness test.
In Sealed enamel subgroups, the adhesive primer was also affected by carbonated water. The adhesive primer was roughened and partially removed in Groups 1–4, and some parts of the etched surface were revealed. In this context, the revealed etched surface had a more important significance in practice, because of demineralization. In the Sealed enamel subgroup, calcium ions had little effect on the protective effect of the adhesive primer.
During orthodontic treatment with fixed appliances, frequent intake of carbonated water could increase the risk of erosion of enamel around the brackets. Erosion of enamel around the brackets, which had been etched for bonding,
2324 could cause dental caries and decrease the retention of the appliances. Even though the etched enamel had been sealed with adhesive material, carbonated water could strip off the adhesive and reveal the etched enamel. Therefore, clinicians should instruct orthodontic patients that carbonated water has negative effects on teeth, especially those with fixed appliances.









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download