Dear Editor,
X-linked Charcot-Marie-Tooth disease type 1 (CMTX1) is a hereditary motor and sensory neuropathy caused by mutations in the gap junction beta-1 (GJB1) gene. Clinically this disease is characterized by slowly progressive wasting and weakness of distal muscles with sensory disturbance.1 We describe the occurrence of amyotrophic lateral sclerosis (ALS) in the proband of a CMTX1 pedigree carrying a known GJB1 mutation who suddenly experienced a rapid clinical decline.
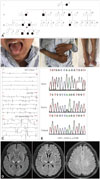
The pedigree displayed a typical X-linked inheritance pattern (Fig. 1A). The proband (III-3) was a 35-year-old man who presented with slowly progressive weakness in the legs that had begun when he was 15 years old. He had gait abnormalities but could walk without an aid. At the age of 33 years he developed weakness of the left hand, followed by both arms. He experienced neck weakness and quadriparesis one year later. Two months before admission he developed dysarthria and dysphagia, followed by difficulties in breathing.
A neurological examination showed severe dysarthria, obvious atrophy and fasciculation of the tongue, and bilateral claw hands and claw feet (Fig. 1B). He had severe muscle wasting and weakness of the neck and limbs (0/5 in all limbs). His deep tendon reflexes were generally diminished, with a bilateral positive palmomental reflex and right positive Babinski sign. Sensations were diminished in the distal limbs. A nerve conduction study revealed severe sensorimotor axonal neuropathy (Supplementary Table 1 in the online-only Data Supplement), EMG showed diffuse acute denervation and chronic reinnervation in the tongue and in the muscles of the left paravertebral T10 and upper and lower limbs (Fig. 1C, Supplementary Table 2 in the online-only Data Supplement). His forced vital capacity was below 70% of predicted. Brain MRI showed an increased symmetrical fluid attenuation inversion recovery (FLAIR) signal intensity in the posterior limb of the internal capsule (Fig. 1D). Screening for ALS and Charcot-Marie-Tooth disease (CMT) related genes (Supplementary Table 3 in the online-only Data Supplement) using Next-Generation Sequencing identified a known hemizygous missense mutation (c.622G>A, p.E208K) of GJB1 (Fig. 1E).2 The symptoms of bulbar palsy and dyspnea continued to progress, and the proband died from respiratory failure 9 months after admission. The clinical features and genetic results for other members of the pedigree are presented in the Supplementary Material (in the online-only Data Supplement).
The pedigree in our study exhibited the typical clinical features of CMTX1. Male patients had a relatively uniform phenotype, presenting within the first 2 decades of life with difficulty in walking, distal lower limbs weakness, and sensory loss. However, after the age of 33 years, the proband suddenly experienced a rapid clinical decline. He developed generalized weakness and soon lost ambulation ability, with subsequent bulbar and respiratory involvement. Upper motor neuron signs also appeared. From a clinical standpoint, a rapidly progressive motor syndrome in CMT should always prompt the search for another diagnosis beyond CMT. Indeed, EMG findings of diffuse acute denervation and chronic reinnervation, upper motor neuron involvement, a symmetrical FLAIR signal intensity in the posterior limb of the internal capsule, and the rapid clinical decline strongly support a diagnosis of ALS. We therefore conclude that the proband developed a CMTX1 neuropathy with superimposed clinically probable ALS.3
CMT type 1A in combination with Kennedy's disease has been reported previously. The association of these two diseases enhances the degree of disability.4 Marchesi et al.5 also reported the occurrence of ALS in a 62-year-old woman affected by the early onset of slowly progressive CMT type 2A. She developed slowly progressive distal limb muscle wasting and weakness, and mild distal leg sensory loss during the first decade. After the age of 60 years she rapidly developed generalized muscle wasting and fasciculation, together with dysarthria and dysphagia. The clinical course of the proband in our study is similar to that of the patient reported by Marchesi et al.5
While the probability is extremely low, the combination of two rare neuromuscular diseases should be considered when unusual symptoms present that cannot be entirely explained by a single disease.
Figures and Tables
Fig. 1
Clinical features of the proband and segregation analysis of the GJB1 p.E208K mutation. A: The pedigree of the proband diagnosed with CMTX1 and ALS. B: The clinical features of the proband, with obvious atrophy tongue and bilateral claw hands and pes cavus. C: Spontaneous activities in an EMG examination in the proband. D: Brain MRI showed increased symmetrical FLAIR signal intensity in the posterior limb of the internal capsule (arrows). E: Segregation analysis of the GJB1 p.E208K mutation. ALS: amyotrophic lateral sclerosis, CMTX1: Charcot-Marie-Tooth disease type 1, FDI: first dorsal interosseous, FLAIR: fluid attenuation inversion recovery, GJB1: gap junction beta-1.
Acknowledgements
The authors thank the patients and their families for their cooperation in this study. This study was supported by the Natural Science Foundation of Fujian Province (grant number 2015J01395) and the National Natural Science Foundation of China (grant number 81671271).
References
1. Wang Y, Yin F. A review of X-linked Charcot-Marie-Tooth disease. J Child Neurol. 2016; 31:761–772.

2. Fairweather N, Bell C, Cochrane S, Chelly J, Wang S, Mostacciuolo ML, et al. Mutations in the connexin 32 gene in X-linked dominant Charcot-Marie-Tooth disease (CMTX1). Hum Mol Genet. 1994; 3:29–34.

3. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.2.261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download