Abstract
Background and Purpose
Methods
Results
Acknowledgements
References
Fig. 2
Studies on the clinical research information system website. *Could not be found via PubMed search.

Table 1
Summary of clinicaltrials.gov

ABI: anoxic brain injury, AD: Alzheimer's disease, AD-MSCs: adipose-derived mesenchymal stem cells, ALS: amyotrophic lateral sclerosis, BM-MSCs: bone marrow mesenchymal stem cells, CI: cerebral infarction, CP: cerebral palsy, DAT: dementia of Alzheimer's type, GDD: global development delay, hUCB-MSCs: human umbilical cord blood mesenchymal stem cells, IS: ischemic stroke, IVH: intraventricular hemorrhage, MSA: multiple system atrophy, MSCs: mesenchymal stem cells, N: no, PD: Parkinson's disease, P-MSCs: placenta mesenchymal stem cells, SC: stem cells, SCI: spinal cord injury, TBI: traumatic brain injury, UCB: umbilical cord blood, Y: yes.
Table 2
Summary of clinical research information system website

| Search term and status | Registration number | Completion date | Results posted | Results listed as published | Published article found | Article had registration match | Published date | Type of SC | PMID | Disease |
|---|---|---|---|---|---|---|---|---|---|---|
| SC | ||||||||||
| Completed | KCT0001429 | 8-2013 | N | Y | Y (review) | N | 8-2013 | hNSPCs | N | HIBI |
| Completed | KCT0000879 | 12-2016 | N | N | Y | Y | 10-2015 | hNSPCs | 26568892 | TSCI |
| Not yet recruiting (recruiting at clinicaltrials.gov) | KCT0000574 | Anticipated 2-2016 | N | Y | Y (protocol) | N | N | MSCs | 24083670 | IS |
| UCB | ||||||||||
| Recruiting (completed at clinicaltrials.gov) | KCT0000950 | Anticipated 8-201559 (completed 7-2017 at clinicaltrials.gov) | N | N | N | N | N | UCB | N | CP |
| Recruiting (completed at clinicaltrials.gov) | KCT0000483 | Anticipated 12-2012 (completed 3-2013 at clinicaltrials.gov) | N | N | N | N | N | Allogeneic UCB | N | CP |
| Recruiting (completed at clinicaltrials.gov) | KCT0000445 | Anticipated 5-2014 (completed 8-2013 at clinicaltrials.gov) | N | N | N | N | N | Allogeneic UCB | N | GDD |
Table 3
Summary of PubMed
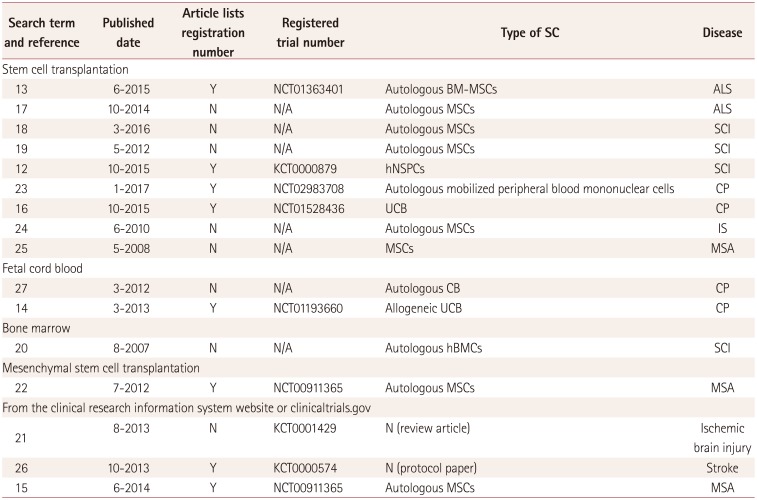
| Search term and reference | Published date | Article lists registration number | Registered trial number | Type of SC | Disease |
|---|---|---|---|---|---|
| Stem cell transplantation | |||||
| 13 | 6–2015 | Y | NCT01363401 | Autologous BM-MSCs | ALS |
| 17 | 10–2014 | N | N/A | Autologous MSCs | ALS |
| 18 | 3–2016 | N | N/A | Autologous MSCs | SCI |
| 19 | 5–2012 | N | N/A | Autologous MSCs | SCI |
| 12 | 10–2015 | Y | KCT0000879 | hNSPCs | SCI |
| 23 | 1–2017 | Y | NCT02983708 | Autologous mobilized peripheral blood mononuclear cells | CP |
| 16 | 10–2015 | Y | NCT01528436 | UCB | CP |
| 24 | 6–2010 | N | N/A | Autologous MSCs | IS |
| 25 | 5–2008 | N | N/A | MSCs | MSA |
| Fetal cord blood | |||||
| 27 | 3–2012 | N | N/A | Autologous CB | CP |
| 14 | 3–2013 | Y | NCT01193660 | Allogeneic UCB | CP |
| Bone marrow | |||||
| 20 | 8–2007 | N | N/A | Autologous hBMCs | SCI |
| Mesenchymal stem cell transplantation | |||||
| 22 | 7–2012 | Y | NCT00911365 | Autologous MSCs | MSA |
| From the clinical research information system website or clinicaltrials.gov | |||||
| 21 | 8–2013 | N | KCT0001429 | N (review article) | Ischemic brain injury |
| 26 | 10–2013 | Y | KCT0000574 | N (protocol paper) | Stroke |
| 15 | 6–2014 | Y | NCT00911365 | Autologous MSCs | MSA |
ALS: amyotrophic lateral sclerosis, BM: bone marrow, CB: cord blood, CP: cerebral palsy, CRIS: clinical research information system, hBMCs: human bone marrow cells, hNSPCs: human neural stem/precursor cells, IS: ischemic stroke, MSA: multiple system atrophy, MSCs: mesenchymal stem cells, N: no, N/A: not available, SC: stem cells, SCI: spinal cord injury, UCB: umbilical cord blood, Y: yes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download