Introduction
Bullous pemphigoid (BP) is an autoimmune disease characterized by subepidermal blistering occurring mainly in elderly people between 60 and 70 years of age
1. Its annual incidence is estimated at between 0.2 and 1.4 per 100,000 person-years
234, and this value will probably increase with the increasing proportion of the elderly in the population. The pathophysiology is characterized by the action of autoantibodies against specific antigens in hemidesmosomes at the epidermal junction
5 causing separation of the epidermis from the dermis.
Thus, the immune response of BP is characterized by the action of circulating autoantibodies of immunoglobulin (Ig)G and C3 against the structural components of the basement membrane zone (BMZ)
67. These autoantibodies have two main targets: antigen 1 (BPAG1, also known as BP230) and antigen 2 (BPAG2, BP180 or collagen type XVII). It is likely that this autoimmunity involves autoreactive T cells that recognize epitopes located in the extracellular region of BP180, mainly in the NC16A ectodomain
89.
There is strong evidence that genetic factors including HLA alleles may promote activation of potentially autoreactive T cells
101112 and different alleles and haplotypes have been identified in different populations.
Caucasian patients with BP were the first to be associated with the allele HLA-DQB1*03:01
13, and it was later associated with Iranian patients
14. However, in the Japanese population, patients with BP were associated with haplotypes HLA-DRB1*04/DQA1*03:01/DQB1*03:02 and DRB1*11:01/DQA1*0505/DQB1*03:02, and individual alleles DRB1*11:01 and DQB1*03:02
15.
However, in the Chinese population with BP
16, there was no statistically significant association with DQB1*03:01, but there was a protective factor for BP in individuals who presented the DRB1*08
loci (DR8) and DRB1*08/DQB1*06. These findings indicate that different HLA class II alleles and haplotypes can influence the genetic susceptibility to BP in different populations.
Therefore, this study was conducted to evaluate the association of HLA alleles (loci A, B, C, DR and DQ) with BP in Brazilian patients, by comparing the prevalence of these alleles with those in a control group.
Go to :

DISCUSSION
BP is an autoimmune-mediated blistering disease affecting the elderly. It is assumed that autoreactive T cell responses to BPAG2 are elicited upon recognition of this antigen bound to the HLA class II region of DQB1 molecule
1718.
This study reports the association of HLA class I (loci A, B and C) and HLA class II (loci DR, DQ) with BP, in Sao Paulo city, southeastern Brazil.
The Brazilian population, one of the most heterogeneous in the world, favors case–control studies with genotyping by allowing a reduction in connection imbalance and thus decreasing the chance of false positives
19.
In the present study, most patients had no association with any external factors: only 1 (5.9%) reported a reaction following the use of amoxicillin and 1 (5.9%) reported a period of major stress preceding the symptoms.
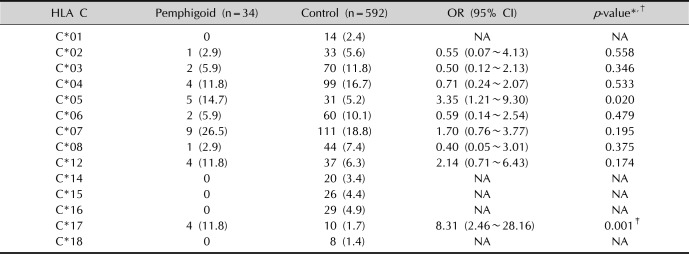
In our study, individuals with HLA C*17 are at approximately eight times higher risk of presenting pemphigoid compared to individuals who do not have this allele (
p <0.001). Although previous studies
1315 did not report an association of BP with class I alleles in other populations, this study has shown an association in Brazilian patients with BP, and this is the only statistically significant HLA class I reported so far.
However, with respect to HLA class II, in Japanese patients, Okazaki et al.
15 demonstrated that, compared with a control group, patients with BP were associated with haplotypes HLA-DRB1*04/DQA1*03:01/DQB1*03:02 (39.1% vs. 11.7%;
p <0.001) and DRB1*11:01/DQA1*05:05/DQB1*03:02 (8.7% vs. 0%;
p<0.0001), and alleles DQB1*03:02 (43.5% vs. 17.2%;
p<0.002) and DRB1*11:01 (17.4% vs. 3.2%;
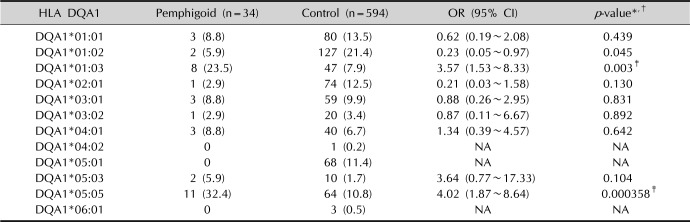
p<0.001). Similarly, in our study, the presence of the allele DQA1*05:05 increased the risk of manifest BP approximately fourfold (
p=0.000358), however, with respect to other Class II genes reported in the study by Okazaki et al.
15, there were no statistically significant differences.
Furthermore, in our study, the allele DQA1*01:03 showed a relative risk approximately 3.57 times (p=0.003) higher in Brazilian individuals with BP. This new association has not been described in the literature yet, thus proving the existence of different alleles in the genesis of pemphigoid, according to the population studied.
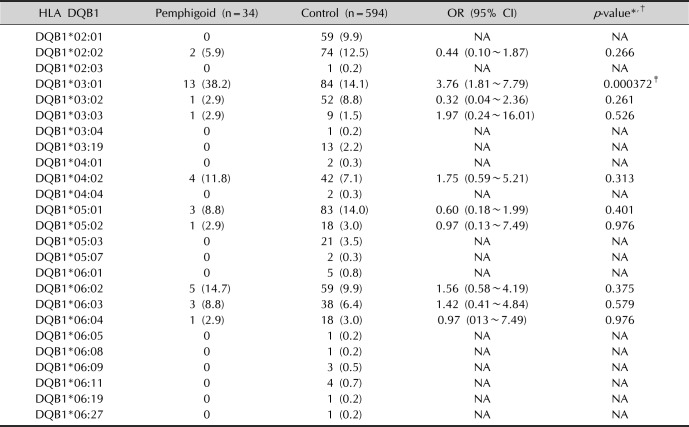
Regarding the allele DQB1*03:01, the study by Delgado et al.
13 showed a significant association in the Caucasian population (35.7% vs. 16.05%,
p=0.005) in all clinical variants of pemphigoid (BP, oral pemphigoid and ocular cicatricial pemphigoid). It is currently the most common allele present in the different populations studied. Similarly, in our study, this allele also showed an approximately 3.76 times greater risk compared to the control group (38.2% vs. 14.1%,
p=0.000372), demonstrating an even more important role in the genesis of this pemphigoid allele.
Just as in the Caucasian population, another study by Esmaili et al.
14 in Iranian patients with BP found a significant association with the HLA-DQB1*03:01 allele (36% vs. 23.6%,
p=0.02), the HLA-DQA1*05:01 allele (45% vs. 33%,
p=0.03) and the HLA-DQB1*04:01 allele (4% vs. 1.6%,
p=0.04) compared with the control group. However, in our study, we found no association with the HLA-DQA1*05:01 and HLA-DQB1*04:01 alleles in BP patients, so HLA-DQB1*03:01 remains the only common allele among Brazilian and Iranian patients.
On the other hand, in a study in Chinese patients
16, there was a protective factor for BP in individuals who presented the DRB1*08 and DQB1*06 alleles (8.04% vs. 15.19% and 1.54% vs. 13.82%, respectively;
p <0.05) compared with controls. However, in our study, we did not find a statistically significant difference in DRB1*08 (2.9% vs. 6.7%,
p =0.398) and DQB1*06 (26.5% vs. 22.1%,
p=0.548) alleles compared with controls in Brazilian patients with BP.
In conclusion, our study demonstrates that alleles HLA C*17, DQB1*03:01, DQA1*01:03, and DQA1*05:05 are associated with BP in Brazilian patients with relative risks of 8.31 (2.46 to 28.16), 3.76 (1.81 to 7.79), 3.57 (1.53 to 8.33), and 4.02 (1.87 to 8.64), respectively.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download