Abstract
Pyoderma gangrenosum (PG) is a rare chronic neutrophilic dermatosis characterized by painful necrotic ulceration. The most common diseases associated with PG are inflammatory bowel disease, certain rheumatologic and hematologic diseases, and malignancy. Here, we describe the case of a 60-year-old man who presented with pruritic and painful erythematous ulcerative macules and patches on both lower extremities, and was diagnosed with PG based on his clinical and histologic features. His PG became exacerbated despite standard therapy with a high-dose systemic steroid in combination with dapsone and cyclosporine. Systemic evaluation of underlying conditions revealed rectal adenocarcinoma at the rectosigmoid junction (T3N0M0), which was completely removed via Hartmann's procedure followed by adjuvant chemotherapy. Two months after anticancer therapy, his PG was completely healed with hypertrophic scarring. Herein, we present the first case of paraneoplastic PG caused by rectal adenocarcinoma in Korea.
Pyoderma gangrenosum (PG) is a rare chronic neutrophilic dermatosis characterized by painful necrotic ulceration. It has been reported to be associated with various disorders123, including inflammatory bowel disease, certain rheumatologic and hematologic diseases, and malignancy. Paraneoplastic PG was first described in 1993 by Duguid et al.4 in 4 patients in association with myeloproliferative malignancy. However, there have been few reports of paraneoplastic PG caused by solid malignant tumors. Here, we present the first Korean case of paraneoplastic PG caused by rectal adenocarcinoma.
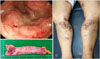
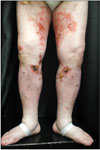
A 60-year-old man visited our dermatologic clinic with a 5-month history of skin eruptions. The skin lesions were localized on the lower extremities as pruritic and painful ulcers accompanied by leg edema (Fig. 1). He had a 3-year history of hypertension. Laboratory examinations revealed negative results for antinuclear antibody and antineutrophil cytoplasmic antibody. To exclude vascular disease, Doppler ultrasonography was performed, which showed no specific findings. Skin biopsy of the right lower leg showed central necrotizing suppurative inflammation with ulceration, demonstrating neutrophilic infiltration with leukocytoclasia and dermolysis in the central part of the lesions without vasculitis. Multiple infections, including mycobacteria, deep fungi, or other bacterial diseases, were excluded based on negative tissue culture results. After ruling out other tentative diagnoses, PG was diagnosed based on his clinical and histopathologic features. The patient received intensive treatment with a high-dose systemic steroid (0.5∼1 mg/kg/d) in combination with dapsone and cyclosporine; however, the ulcerative lesions became aggravated. Systemic evaluation of underlying diseases, including routine laboratory tests, peripheral blood smear, abdominal computed tomography (CT), chest CT, and colonoscopy, was performed. On colonoscopy, a huge ulcerofungating mass at the rectosigmoid junction was detected, which was confirmed as rectal adenocarcinoma by histopathologic examination (Fig. 2A).
PG is characterized by rapidly progressive ulcers with irregular and undermined borders. PG is a rare noninfectious dermal neutrophilia on the spectrum of neutrophilic dermatoses5, which share similar clinical features, such as underlying disorders, a tendency for pathergy, and therapeutic response. Lesions can be classified morphologically as ulcerative, bullous, pustular, or vegetative6. To establish diagnosis of PG, clinicians should exclude all other possible associated diseases6. Approximately half of PG cases are known to be associated with underlying systemic diseases, such as rheumatoid arthritis, inflammatory bowel disease, or myeloproliferative disorders7. However, there have been several reports of PG associated with solid malignancies89101112131415. Although the exact mechanisms by which solid tumors induce PG are unknown, abnormal immune surveillance, such as neutrophilic dysfunction, defects in chemotaxis, or hyperreactivity, may play a role as in PG associated with other conditions16. Several cases of PG in relation to colorectal carcinoma have been reported (Table 1)11131415. In most of these reports, patients presented with a solitary ulcer in various locations111314. On the other hand, Shahi and Wetter15 reported PG associated with solid organ malignancies, including a case of recurrent PG presenting with numerous ulcers on the legs. Owing to the patient's history of ulcerative colitis, it is uncertain whether the lesions were related to ulcerative colitis or rectal cancer.
Our patient presented with multiple ulcers on the lower extremities, and systemic evaluation of underlying diseases revealed rectal adenocarcinoma. To our knowledge, there has been no report of PG presenting as multiple ulcers in relation to primary colorectal carcinoma in a Korean patient. This case was confirmed as paraneoplastic PG caused by progressive colorectal cancer based on the following findings: (1) the lesions did not respond to standard immunosuppressive agents used to treat PG; (2) the refractory lesions healed dramatically after complete removal of the tumor; and (3) there was no recurrence of PG after the solid malignancy was removed during our 3-year follow-up.
In conclusion, we reported the first Korean patient with paraneoplastic PG manifesting as multiple leg ulcers caused by rectal adenocarcinoma. From our experience, and because colorectal cancer has become a common malignancy worldwide, intensive evaluation to detect underlying solid malignancies is required, especially in cases of refractory PG that do not respond to conventional therapies.
Figures and Tables
 | Fig. 2(A) Colonoscopic image, showing an ulcerofungating mass at the rectosigmoid junction. (B) Hartmann's procedure was performed for resection of colon cancer. A 5.5×4-cm rectal adenocarcinoma (T3N0M0) was found. (C) Clinical photograph, showing completely healed pyoderma gangrenosum lesions with hypertrophic scarring after the fourth rounds of adjuvant chemotherapy. |
Table 1
Previously reported cases of PG associated with colorectal malignancies

| Study | Sex/age (yr) | PG subtype | PG location | The number of lesions | Solid organ cancer | Timing of PG | Other associated systemic disease |
|---|---|---|---|---|---|---|---|
| Bunte et al.11 (2008) | M/53 | Ulcerative (classic) | Right scapula (injury while guardening) | Solitary | Adenocarcinoma of the sigmoid colon | 4 months before diagnosis of malignancy | None |
| Foley and Laing13 (2015) | F/70 | Ulcerative (classic) | Upper back | Solitary | Colon cancer | NA | None |
| Sakai et al.14 (2006) | F/70 | Peristomal | Peristoma | Solitary | Rectal cancer | 40 days after ileostomy for the rectal cancer | None |
| Shahi and Wetter15 (2015) | M/36 | Ulcerative (classic) | Lower extremities | Multiple | Metastatic rectosigmoid carcinoma to lungs | Synchronous | Ulcerative colitis |
| Present study | M/60 | Ulcerative (classic) | Lower extremities | Multiple | Rectal adenocarcinoma | 6 months before diagnosis of malignancy | None |
References
1. Crowson AN, Mihm MC Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003; 30:97–107.

2. Magro CM, Crowson AN. A distinctive vesiculopustular eruption associated with hepatobiliary disease. Int J Dermatol. 1997; 36:837–844.

3. Lee JI, Park HJ, Lee JY, Cho BK. A case of pyoderma gangrenosum with ulcerative colitis treated with mesalazine. Ann Dermatol. 2010; 22:422–425.

4. Duguid CM, O'Loughlin S, Otridge B, Powell FC. Paraneoplastic pyoderma gangrenosum. Australas J Dermatol. 1993; 34:17–22.

5. Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004; 43:790–800.

6. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996; 34:395–409.

7. Mlika RB, Riahi I, Fenniche S, Mokni M, Dhaoui MR, Dess N, et al. Pyoderma gangrenosum: a report of 21 cases. Int J Dermatol. 2002; 41:65–68.

8. Gateley CA, Foster ME. Pyoderma gangrenosum of the breast. Br J Clin Pract. 1990; 44:713–714.
9. Gallo R, Parodi A, Rebora A. Pyoderma gangrenosum in a patient with gastric carcinoma. Int J Dermatol. 1995; 34:713–714.

10. Basille W, Dompmartin A, Lorier E, Sautreuil B, Girardot PM, Leroy D. Pyoderma gangrenosum. Association to acinar cell carcinoma of the parotid gland. Ann Dermatol Venereol. 1992; 119:381–383.
11. Bunte C, Popp-Habeler J, Mischer P, Tuppy H, Haidenthaler A, Knoflach P, et al. Concomitant manifestation of pyoderma gangrenosum and colorectal carcinoma. Scand J Gastroenterol. 2008; 43:756–758.

12. Regnier-Rosencher E, Bizet N, Méry L. Pyoderma gangrenosum associated with renal carcinoma. J Am Acad Dermatol. 2011; 64:1208–1211.

13. Foley CC, Laing M. Paraneoplastic pyoderma gangrenosum successfully treated with minocycline and low-dose steroids. J Eur Acad Dermatol Venereol. 2015; 29:184–185.

14. Sakai H, Otsubo S, Iizuka H. Peristomal pyoderma gangrenosum associated with rectal adenocarcinoma. J Dermatol. 2006; 33:68–70.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download