INTRODUCTION
Squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are the most common nonmelanoma skin cancers, and their incidences continue to increase
1. The clinical features of SCC and BCC are well known, and, in most cases, proper diagnosis can be easily made on the basis of clinical information. SCC presents as firm, flesh-colored keratotic papules or plaques and smooth nodules. A thick cutaneous horn and ulceration may accompany these lesions. Features that suggest BCC are translucency, ulceration, telangiectasias, pigmentation, and a rolled border. However, as both carcinomas commonly occur in sun-exposed areas such as the head and neck
2, and sometimes the clinical presentations of these cancers are ambiguous, confusions may arise and it may be difficult to make a proper diagnosis. Therefore, in this study, we aimed to determine what cause confusion for physicians in making the clinical diagnosis, and lead to misdiagnosis of SCC as BCC or
vice versa. We also evaluated the effect of dermoscopy to make proper diagnoses for these lesions.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB no. ED17109). The IRB waived the requirement for written informed consent because this research involves no more than minimal risk to the subjects. We reviewed data for recent three years and selected all cases confirmed as BCC or SCC by using clinical and dermoscopic photographs. We checked the first clinical impressions of these cases and collected those cases whose clinical impressions were inverse to the final diagnosis.
Six dermatologists (three board-certified and three resident dermatologists) were presented with the clinical and dermoscopic images of inversely diagnosed cases in random order. These dermatologists evaluated the images and made a diagnosis for each image based on the clinical or dermoscopic findings (scaling, pigmentation, ulceration, telangiectasia, rolled border, and translucency for clinical evaluation and vascular pattern; scaling, ulceration, white structures, leaf-like areas, blue-gray ovoid nests and globules, and spoke-wheel areas for dermoscopic evaluation;
Table 1). They checked multiple findings to identify what information from the photograph led to the diagnosis. All data were collected and analyzed. Generalized estimating equation (GEE) analysis was used to compare results, and the level of significance in this study was set at a
p-value of <0.05 with 95% confidence limits. Analyses were performed with IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
Table 1
Findings suggestive of clinical diagnosis

|
Clinical |
Scaling, pigmentation, ulceration, telangiectasia, rolled border, translucency |
|
Dermoscopic |
|
|
SCC |
Vascular morphology (linear-irregular, hair-pin, dotted, glomerular), vascular pattern (monomorphous/polymorphous), keratin formation, ulceration, white structures |
|
BCC |
Arborizing vessels, leaf-like areas, large blue-gray ovoid nests or blotches, multiple blue-gray globules, spoke-wheel areas, focal ulceration |

Go to :

RESULTS
A total of 154 cases with histopathologic diagnosis of BCC or SCC were reviewed. Among them, 141 cases were clinically diagnosed correctly with the proper clinical impression; however, 13 cases were clinically misdiagnosed with the inverse impression. For inversely diagnosed cases, nine cases were SCCs clinically misdiagnosed as BCC and four cases were BCCs clinically misdiagnosed as SCC. Among four cases of misdiagnosed BCC, two cases were confirmed to be basosquamous carcinoma (BSC) in histopathologic examination. Two of SCCs and one of BCCs have history of previous laser therapy (
Fig. 1).
 | Fig. 1Flowchart of the study. Of a total 154 squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) cases, 13 cases that were misdiagnosed clinically with the inverse impression were enrolled in this study. Lesions with a previous laser treatment did not show improved diagnostic accuracy with dermoscopy.
|
Clinical features referred to diagnoses of each case by six evaluators are indicated in
Table 2. Three cases out of 13 cases were fairly well diagnosed based on clinical photographs (cases 2, 4, and 12), and all these cases have no problem in making diagnoses with dermoscopic photos (
Table 3). However, in most cases, 10 out of 13 cases, there were difficulties to make proper diagnoses (half and more of six raters made misdiagnoses) clinically and many of them showed improved diagnostic accuracy by dermoscopy, although some cases did not (cases 6, 8, 9, and 10).
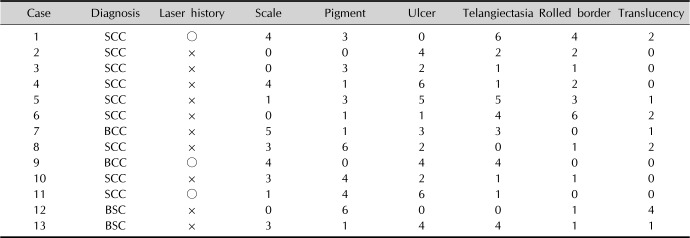
Table 2
Clinical features referred to each diagnosis by six evaluators
|
Case |
Diagnosis |
Laser history |
Scale |
Pigment |
Ulcer |
Telangiectasia |
Rolled border |
Translucency |
|
1 |
SCC |
○ |
4 |
3 |
0 |
6 |
4 |
2 |
|
2 |
SCC |
× |
0 |
0 |
4 |
2 |
2 |
0 |
|
3 |
SCC |
× |
0 |
3 |
2 |
1 |
1 |
0 |
|
4 |
SCC |
× |
4 |
1 |
6 |
1 |
2 |
0 |
|
5 |
SCC |
× |
1 |
3 |
5 |
5 |
3 |
1 |
|
6 |
SCC |
× |
0 |
1 |
1 |
4 |
6 |
2 |
|
7 |
BCC |
× |
5 |
1 |
3 |
3 |
0 |
1 |
|
8 |
SCC |
× |
3 |
6 |
2 |
0 |
1 |
2 |
|
9 |
BCC |
○ |
4 |
0 |
4 |
4 |
0 |
0 |
|
10 |
SCC |
× |
3 |
4 |
2 |
1 |
1 |
0 |
|
11 |
SCC |
○ |
1 |
4 |
6 |
1 |
0 |
0 |
|
12 |
BSC |
× |
0 |
6 |
0 |
0 |
1 |
4 |
|
13 |
BSC |
× |
3 |
1 |
4 |
4 |
1 |
1 |

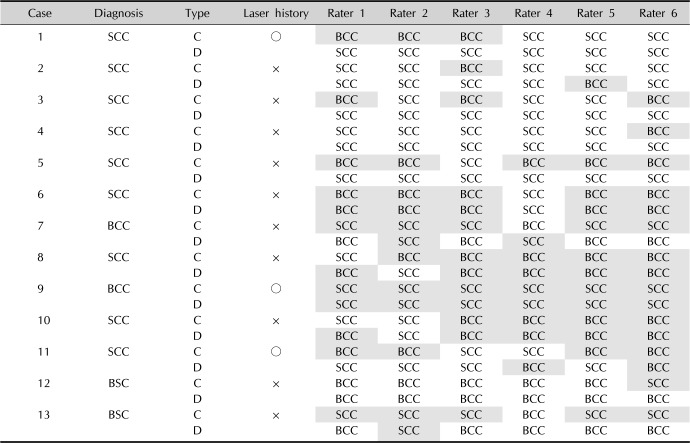
Table 3
Diagnoses by six evaluators based on photographs
|
Case |
Diagnosis |
Type |
Laser history |
Rater 1 |
Rater 2 |
Rater 3 |
Rater 4 |
Rater 5 |
Rater 6 |
|
1 |
SCC |
C |
○ |
BCC |
BCC |
BCC |
SCC |
SCC |
SCC |
|
D |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
2 |
SCC |
C |
× |
SCC |
SCC |
BCC |
SCC |
SCC |
SCC |
|
D |
SCC |
SCC |
SCC |
SCC |
BCC |
SCC |
|
3 |
SCC |
C |
× |
BCC |
SCC |
BCC |
SCC |
SCC |
BCC |
|
D |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
4 |
SCC |
C |
× |
SCC |
SCC |
SCC |
SCC |
SCC |
BCC |
|
D |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
5 |
SCC |
C |
× |
BCC |
BCC |
SCC |
BCC |
BCC |
BCC |
|
D |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
6 |
SCC |
C |
× |
BCC |
BCC |
BCC |
SCC |
BCC |
BCC |
|
D |
BCC |
BCC |
BCC |
SCC |
BCC |
BCC |
|
7 |
BCC |
C |
× |
SCC |
SCC |
SCC |
BCC |
SCC |
SCC |
|
D |
BCC |
SCC |
BCC |
SCC |
BCC |
BCC |
|
8 |
SCC |
C |
× |
SCC |
BCC |
BCC |
BCC |
BCC |
BCC |
|
D |
BCC |
SCC |
BCC |
BCC |
BCC |
BCC |
|
9 |
BCC |
C |
○ |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
D |
SCC |
SCC |
SCC |
SCC |
SCC |
SCC |
|
10 |
SCC |
C |
× |
SCC |
SCC |
BCC |
BCC |
BCC |
BCC |
|
D |
BCC |
SCC |
BCC |
BCC |
BCC |
BCC |
|
11 |
SCC |
C |
○ |
BCC |
BCC |
SCC |
SCC |
BCC |
BCC |
|
D |
SCC |
SCC |
SCC |
BCC |
SCC |
BCC |
|
12 |
BSC |
C |
× |
BCC |
BCC |
BCC |
BCC |
BCC |
SCC |
|
D |
BCC |
BCC |
BCC |
BCC |
BCC |
BCC |
|
13 |
BSC |
C |
× |
SCC |
SCC |
SCC |
BCC |
SCC |
SCC |
|
D |
BCC |
SCC |
BCC |
BCC |
BCC |
BCC |

GEE analysis showed scale, pigmentation and rolled border are meaningful clinical findings for correct diagnosis (p=0.031, 0.009, and 0.037, respectively). This means if a lesion is accompanied with scale without pigmentation or rolled border, it is favoring feature for SCC, but a lesion with pigmentation and rolled border without scale has more probability for BCC. In contrast, ulceration, telangiectasia and translucency were not helpful for making a proper diagnosis clinically. Diagnostic accuracy was improved with dermoscopy. Overall odds ratio (OR) for correct diagnosis was 2.857 when dermoscopic photos are used for diagnosis compared to clinical photos (p=0.0049). When it comes to individual diagnosis, OR for SCC was 2.483 (p=0.052) and OR for BCC was 4.048 (p=0.017). In dermoscopic findings, blue-gray nests and globules, arborizing vessels, and ulceration were dominant features for BCC, whereas keratin formation, white structures, and a vascular morphology (linear-irregular) were frequently attributed to SCC.
Go to :

DISCUSSION
SCCs and BCCs are the most common skin cancers. Both carcinomas are locally invasive and rarely metastasize; however, SCCs have a higher tendency to metastasize than BCCs
3, and discerning between the two carcinomas is important to plan proper management. SCC clinically manifests as firm, erythematous, keratotic papules or plaques with ulcer and occasional pigmentation. Although its dermoscopic features are not well established, scaling and white structureless areas are common in SCC, and variable blood vessels—irregular round or coiled, looped, serpentine, branched, or polymorphic morphology—are observed
45. BCC usually develops on sun-exposed areas with translucency, telangiectasias, and ulceration. A rolled border, namely rodent ulcer, caused by central necrosis is one of the characteristic features of BCC. The classic dermoscopic patterns for BCC are well known (arborizing telangiectasia, large blue-gray ovoid nests, multiple blue-gray globules, maple leaf-like areas, spoke-wheel areas, and ulceration), and its diagnostic sensitivity is reported to be >95%
6.
We confirmed that most SCCs and BCCs were properly diagnosed on the basis of clinical features with no difficulty, and only 13 out of 154 cases were misdiagnosed clinically. For 3 cases (cases 2, 4, and 12) of these misdiagnosed ones, most of evaluators made the proper diagnosis with clinical photos and these cases might have been correctly diagnosed initially had the physicians observed the lesion carefully at first visit. Clinically, SCCs had a higher tendency to be misdiagnosed as BCC than
vice versa. Because BCCs are more common than SCCs, it is possible that physicians may have a tendency to frequently make a diagnosis of BCC; however, we found that some SCCs were misdiagnosed as BCCs when pigmentation or a rolled border—findings favoring for BCC—are accompanied (
Fig. 2). For these lesions dermoscopy did not have much effect on correcting diagnoses in some cases (cases 6, 8, and 10), however, for other misdiagnosed SCCs, dermoscopic findings such as scaling, white structures, or an uncharacteristic vessel pattern revealed to improving diagnostic accuracy (cases 1, 3, 5, and 11). It was relatively rare that BCCs were misdiagnosed as SCC and the evaluators also seemed to be confused in making the proper diagnosis for most of these cases. Pigmentation and rolled border were meaningful findings for BCC, but if scaling was present, BCC was misdiagnosed as SCC. In these cases, dermoscopy was helpful to correct diagnosis (case 7 and 13), but in a case, previous laser therapy caused loss of pigmentation in BCC but scale and vessels (
Fig. 3). This alteration of intrinsic pattern of BCC made the dermoscopic effect useless. Clinical features such as ulceration and telangiectasia were not helpful in discriminating between SCC and BCC as they are found commonly in both.
 | Fig. 2Clinical and dermoscopic photographs of squamous cell carcinoma misdiagnosed as basal cell carcinoma. Ulcer surrounded by rolled border and arborizing vessel (A, C) in case 6, and pigmentation with telangiectasia and ulceration (B, D) in case 8 are seen.
|
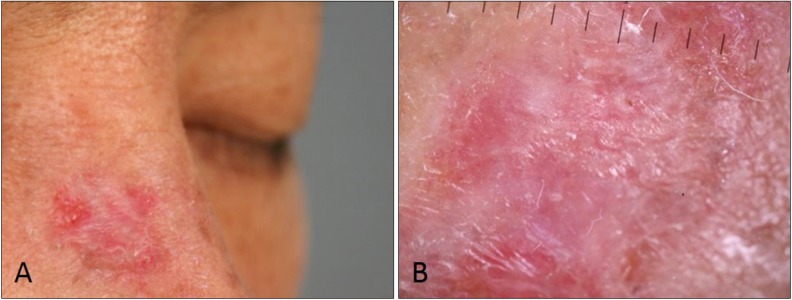 | Fig. 3Clinical and dermoscopic photographs of basal cell carcinoma (BCC) clinically misdiagnosed as squamous cell carcinoma. There are no pigmentations but some scales and vessels are present (A). Dermoscopy shows only keratin without BCC patterns such as blue-gray globules, arborizing vessels, and spoke-wheel areas, may be result of previous laser therapy (B).
|
Contrary to Western cases, most of the BCCs reported in Koreans are of the pigmentation type
7. Therefore, pigmentation was a meaningful finding in the diagnosis of BCC; however, if pigmentation was present in an SCC, it certainly led to a misdiagnosis as BCC. In contrast, if pigmentation was not present in BCC, it also led to a misdiagnosis as SCC. This may explain why SCCs are more frequently misdiagnosed as BCCs than
vice versa, despite the lower occurrence rate of SCCs. Furthermore, our results show that a previous laser treatment may result in loss of pigmentation in BCCs or may cause pigmentation in SCCs, which causes confusion in making the correct diagnosis.
The so-called rodent ulcer is a pathognomonic sign of BCC, which is characterized by a slowly enlarging ulcer surrounded by a rolled border. Not surprisingly, when a rolled border was present in SCC (
Fig. 2), it led to a misdiagnosis as BCC and BCCs without rolled border were misdiagnosed as SCCs. This may be explained by the fact that SCC sometimes has a similar feature of an ulcer surrounded by an elevated, indurative border. Therefore, an ulcer with a rolled border can be found in both BCC and SCC, which causes confusion in the diagnosis.
Overall, dermoscopy is expected to increase the accuracy of diagnosis, as it can evaluate the micro features of carcinomatous lesions, and it was more effective for BCC (2.48 vs. 4.05 in OR). However, dermoscopy showed limitations in ambiguous cases like those in this study (cases 6, 8, 9, and 10). Obviously, the small number of cases and the lack of physician expertise in dermoscopy might have affected the results; however, some cases did not show typical dermoscopic features, especially deformed lesions that were previously treated with laser therapy.
In cases of BSC, making the accurate diagnosis is difficult and can be accomplished only after biopsy. BSC is a rare epithelial neoplasm that is considered as an aggressive type of BCC with ambiguous features (showing features of both SCC and BCC simultaneously). Therefore, considering the rarity, it is not strange that two cases of BSC are misdiagnosed as SCC in this study. There are no specific clinical features to distinguish BSC from other neoplasms. Dermoscopy of BSC did not show characteristic findings and led to a diagnosis of BCC or SCC according to histopathologic findings (
Fig. 4).
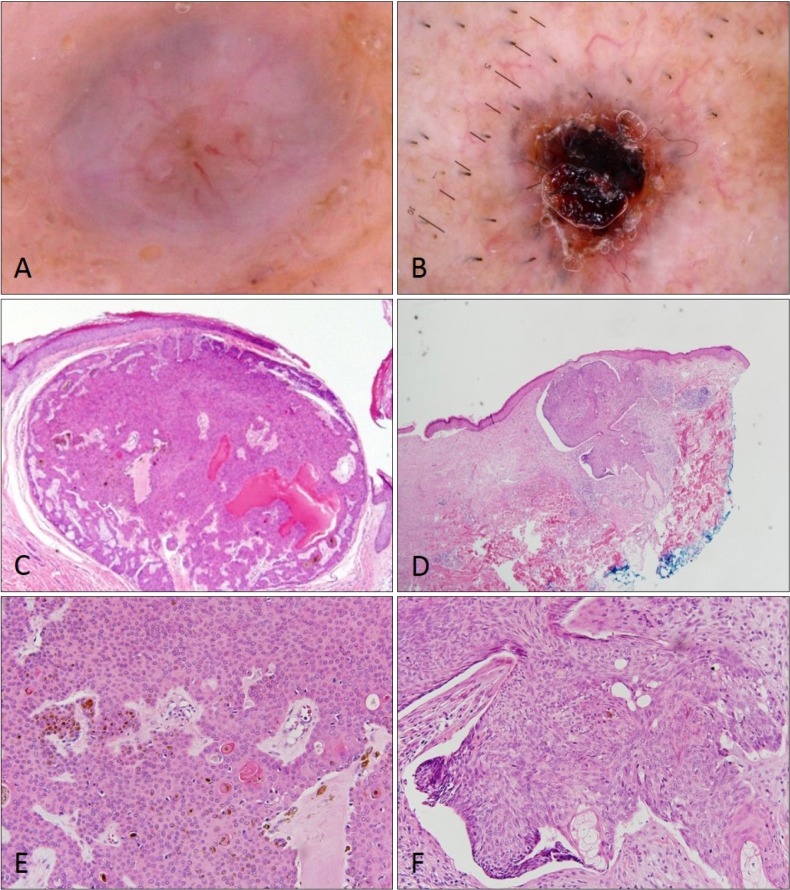 | Fig. 4Dermoscopic and histopathologic findings of basosquamous cell carcinoma. Basosquamous cell carcinoma appeared as basal cell carcinoma or squamous cell carcinoma according to histopathologic findings. Arborizing vessels with hyperkeratosis (A, C, E) and ulceration with telangiectasia (B, D, F) are seen (H&E; C, D: ×40, E, F: ×100).
|
This study has limitation because enrolled cases are not enough in number. We think more cases of ambiguous form are needed to analyze. By comparing typical lesions with distorted ones and making effort to correlate the findings with histopathology, we may get some findings to increase reliability. This can be applied to other confusing or ambiguous lesions which is changed from original appearance, for example, wart vs. seborrheic keratosis, melanoma vs. laser treated pigmented nevus and lentigo maligna vs. pigmented actinic keratosis, etc.
In conclusion, on the basis of clinical impressions, SCC has a higher tendency to be misdiagnosed as BCC than vice versa. However, in cases of ambiguous lesions, pigmentation and a rolled border are factors causing misdiagnosis of SCC as BCC. BCC may be misdiagnosed as SCC when it is accompanied by scaling or a lack of pigmentation due to a previous laser treatment. Ulceration and telangiectasia have no ability to discriminate between SCC and BCC because they contribute to the diagnosis of both carcinomas, and contributed as confusing factors for proper diagnosis. Dermoscopy has the ability to correct a misdiagnosis; however, it seems to have a limitation for some ambiguous carcinomas, especially for previously laser-treated lesions.
Go to :












 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download