INTRODUCTION
In dermatology, ablative laser and radiofrequency (RF) surgery have been used to replace conventional surgery to remove warts, seborrheic keratoses, various nevi, and benign tumors like hemangiomas, and neurofibromas.
Ablative lasers, the CO
2 laser, the 1,444 nm long-pulsed neodymium:yttrium-aluminum-garnet (LP Nd:YAG) laser, and RF emitting electrosurgical units induce a therapeutic effect by heat energy. Infrared light and high-frequency alternating current for laser and RF device, respectively, are converted to heat diffused within the tissues of the operative site. This heat diffusion promotes hemostasis and significantly reduces the bacterial population at the operative site, thus minimizing the risk of intraoperative contamination
1.
However, heat diffusion through the operative site results in pain during the procedure and functional damage, as well as potential scarring secondary to permanent tissue transformation. Lateral thermal injury is determined by a number of variables such as hardness and other characteristics of target tissue, as well as wavelength, power, irradiation mode, power density, and frequency of the laser and RF systems utilized.
To enhance therapeutic results, minimize complications and obtain satisfactory cosmetic outcomes, it is important to understand the characteristics of observed histopathologic changes. Although several studies mention histopathologic changes following thermal injury in experiments performed in vivo and in vitro, it is hard to find studies showing extent of damage using cell death as an indicator.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay is most widely used to detect DNA damage
in situ2. It is not limited to the detection of apoptotic cells: it can also be used to identify necrotic cell death associated with non-apoptotic events such as exposure to toxic compounds and other physical insults
3.
Therefore, this preliminary study aims to evaluate histopathological changes following in vivo thermal damage generated by CO2 laser, 1,444 nm LP Nd:YAG laser and RF emitting electrosurgical unit using TUNEL assay.
Go to :

DISCUSSION
Several studies compare CO
2 laser and RF emitting electrosurgical units during tissue collection and surgery. There is conflicting evidence in studies comparing the lateral thermal injury, level of coagulative necrosis, fibrin deposition, post-operative adhesion, re-epithelialization, post-operative pain and post-operative hemorrhage associated with these treatment modalities
14567891011.
Although several pathologic techniques have been developed to evaluate non-viable cells in tissue sections, there is no single method which is widely accepted with sufficient reliability. TUNEL assay remains the most widely used technique to quantify apoptosis with sensitivity between 60% to 90%
12. However, the most important drawback of this assay is that it cannot discriminate apoptotic from necrotic cells. Hence we first attempted supravital stain which identifies nicotinamide adenosine dinucleotide phosphate (NADPH). However, this method was too expensive and did not yield consistent result. For these reasons, we decided to use TUNEL assay in order to assess non-viable cells after thermal ablation.
According to the findings of our studies using the TUNEL assay, CO
2 laser resulted in more extensive and scattered appearance of cell death area than RF emitting electrosurgical unit. Because both devices are operator-dependent and cannot be standardized, comparison of these methods in clinical settings presents a challenge. Compared to CO
2 lasers, RF emitting electrosurgical units are less expensive, do not require eye protection, and are more readily available
13.
A 1,444 nm LP Nd:YAG laser has a high affinity to fat and water and has thus been primarily used for laser liposuction procedures
14. Now, this laser is being used to treat various conditions such as axillary bromhidrosis
1516, skin laxity
1718, lipoma
19, neurofibroma
20, digital mucoid cysts
21, and xanthelasmata.
In our study, 1,444 nm LP Nd:YAG laser resulted in smaller vaporized tissue volume and more extensive necrosis and coagulation compared with CO2 laser and RF device. As a pulsed laser, the 1,444 nm LP Nd:YAG laser induces larger accumulated energy, as in doing so it is thought to cause broader damage. In addition, changes in the subcutaneous layer were clearly observed in comparison to the other equipment.
The therapeutic effects from above devices are not only due to thermal effect, but also due to other factors like energy source and specific wavelength. However, in comparison based on the same energy intensity, the volume of vaporization was largest with the CO2 laser. Area of cell death area identified by TUNEL assay, when arranged from widest to narrowest, was 1,444 nm LP Nd:YAG laser, CO2 laser, and RF emitting electrosurgical unit.
TUNEL assay showed histopathologic changes resulting from the various intensities and types of damage that otherwise couldn't be detected by H&E staining. We divided the thermal effects of ablative laser and RF irradiation to tissue into four zones and visualized it as a diagram (
Fig. 2). The four zones are composed of vaporization; irreversible tissue damage that appears as cell death and necrosis in the TUNEL assay; reversible tissue damage that appears as denaturation with H&E staining with the absence of necrosis in the TUNEL assay; and, hyperthermia.
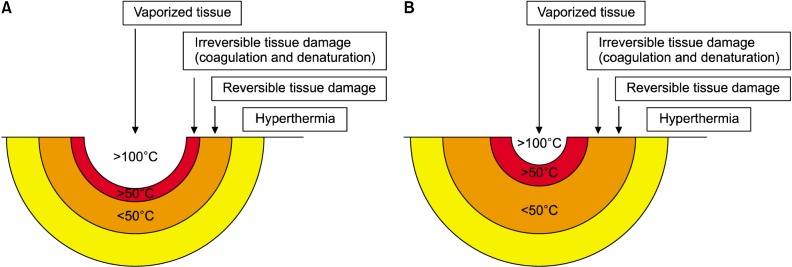 | Fig. 2Thermal effects of ablative laser and radiofrequency irradiation. (A) CO2 laser and radiofrequency emitting electrosurgical unit. (B) 1,444 nm long-pulsed neodymium:yttrium-aluminum-garnet (LP Nd:YAG). 1,444 nm LP Nd:YAG laser resulted in smaller vaporized tissue volume and more extensive necrosis and coagulation compared with CO2 laser and radiofrequency device.
|
However when 1,444 nm LP Nd:YAG laser is applied not to normal skin but to tumors such as neurofibromas, xanthelasmata, angiomas, and pyogenic granulomas, thermal energy is confined within the masses. Consequentially, coagulation and denaturation are predominantly observed while vaporization is minimally observed. Therefore, the characteristics of the treated tissue need to be considered when applying the results of this study clinically.
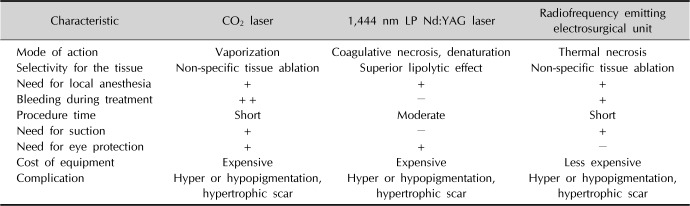
In addition, the main therapeutic mechanisms of action and strengths and weaknesses of CO
2 laser, 1,444 nm LP Nd:YAG laser, and RF device were tabulated (
Table 1). When removal of a skin lesion is desired using a modality that replaces surgical treatment, choosing a treatment method in consideration of these points is considered to be helpful to minimize side effects and obtain a good cosmetic result.
Table 1
Comparison of the characteristics and advantages/disadvantages of the three treatments modalities
|
Characteristic |
CO2 laser |
1,444 nm LP Nd:YAG laser |
Radiofrequency emitting electrosurgical unit |
|
Mode of action |
Vaporization |
Coagulative necrosis, denaturation |
Thermal necrosis |
|
Selectivity for the tissue |
Non-specific tissue ablation |
Superior lipolytic effect |
Non-specific tissue ablation |
|
Need for local anesthesia |
+ |
+ |
+ |
|
Bleeding during treatment |
++ |
− |
+ |
|
Procedure time |
Short |
Moderate |
Short |
|
Need for suction |
+ |
− |
+ |
|
Need for eye protection |
+ |
+ |
− |
|
Cost of equipment |
Expensive |
Expensive |
Less expensive |
|
Complication |
Hyper or hypopigmentation, hypertrophic scar |
Hyper or hypopigmentation, hypertrophic scar |
Hyper or hypopigmentation, hypertrophic scar |

There are several limitations to this research. First, in this experiment, rat skin, which has thinner overall thickness than human skin, was used. In particular, the stratum corneum is sparser and the epidermis is thin, so sensitivity to thermal damage is likely to be higher. Currently, there is no experimental model that closely represents human skin apart from the skin of other mammals; development of other models is necessary.
Second, normal skin without pathological lesions was used. When laser and RF devices are applied to human skin, treatment response can vary because treated tissue has various characteristics; for example, acanthotic lesions such as seborrheic keratoses, high-water content tumors like angiomas and neurofibromas, and high-lipid content tumors like xanthelasmata. However, since efficacy and side effects of treatment are influenced significantly by the range of cryogenic and thermal effects to the normal tissue surrounding the lesion, the results of this experiment should be considered for application to practice.
Third, it was difficult to distinguish between apoptotic and necrotic cells using the TUNEL assay. Hereafter, we will consider other feasible stains or serial biopsies on same condition to characterize apoptosis and necrosis.
Fourth, statistical comparison across conditions presented a challenge, as research was not conducted utilizing large sample sizes within each setting.
In conclusion, use of the TUNEL assay made it possible to observe tissue damage at the cellular level that was previously unidentifiable with conventional H&E staining. Thermal damage was variable depending on the device and energy used. Histopathologic analysis of acute cellular damage induced by various devices can be implemented in clinical practice. It could assist in predicting the reaction of human tissues to the planned treatment modality (laser, and RF emitting electrosurgical unit), leading to an optimal treatment outcome. In the future, further studies of cell death with more variable conditions and samples are a requisite.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download