Abstract
Background
Bacterial skin infections occur secondarily in conditions involving a vulnerable skin barrier such as atopic eczema, as well as primarily such as impetigo. They are mainly caused by Staphylococcus aureus and Streptococci. Recently, the prevalence of methicillin-resistant S. aureus has been increasing.
Objective
To determine the characteristics of community-acquired bacterial skin infections, to observe their antibiotic susceptibility patterns, and to evaluate factors contributing to the treatment response.
Methods
We retrospectively reviewed outpatients under 30 years old from 2010 to 2015, from whom we had taken skin swabs for antibiotic susceptibility testing. We collected clinical and microbiological characteristics from the medical records.
Results
We evaluated the culture results of 197 patients and reviewed their medical records. Overall, 86.3% (n=170) of the patients responded to the initial treatment regimen. S. aureus was the most commonly isolated pathogen (52.6%) and showed a high resistance rate to penicillin (90.9%) and oxacillin (36.3%). In the multivariable logistic regression analysis, resistance to 3 or more antibiotics (p=0.044), culture amounts described as “many” (p=0.040), and non-systemic antibiotic use (p<0.001) were significantly associated with lower treatment response. However, methicillin resistance was not associated with lower treatment response both in univariable and multivariable analyses.
Conclusion
Among young patients, S. aureus was the most predominant pathogen present in bacterial skin infections. Resistance to high numbers of antibiotics and the use of non-systemic antibiotics were associated with lower treatment response. First-generation cephalosporins may be the most effective first-line empirical regimen for bacterial skin infections treated in outpatient settings, regardless of methicillin resistance.
Skin infections are among the most common disorders found in community and hospital environments. These can present in a variety of forms, ranging from limited superficial infections that are controlled by treatment with topical antibiotics to severe infections of deep tissues that can lead to death if the patient is not appropriately treated. Staphylococcus aureus and Streptococcus species are the most commonly isolated causative organisms of skin infections; thus, treatment is prescribed empirically to cover these two pathogens. However, antibiotic resistance has been increasing due to frequent use of antibiotics and the increased number of nursing facilities. In particular, the emergence of methicillin-resistant S. aureus (MRSA) strains resistant to beta-lactam antibiotics has become a problem. In addition, the proportion of methicillin-resistant coagulase-negative Staphylococci (MRCoNS) has been increasing. Clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) are recommended for outpatient treatment of skin infections because of their activity against many MRSA strains12. It is known that community-acquired MRSA isolated from skin infections are resistant to semi-synthetic penicillins such as first-generation cephalosporins, and are almost always sensitive to TMP-SMX3.
However, most previous studies on antibiotic therapy in skin infections mainly focused on treating skin abscesses14 and cellulitis5. Thus, it is difficult to apply currently recommended antibiotic regimens to other bacterial skin infections. Bacterial skin infections other than abscesses and cellulitis often require antibiotic treatment. In particular, secondary infections can develop in atopic dermatitis; these superficial bacterial infections are common and require antibiotic treatment67. However, the appropriate management of superficial bacterial infections and secondary infections in eczema is unclear.
In this study, we aimed to investigate the clinical and microbiological characteristics of patients diagnosed with bacterial skin infections. Additionally, we analyzed predictors of treatment response.
We retrospectively reviewed the medical records of patients from whom we obtained bacterial cultures of skin lesions, those who visited the Department of Dermatology at SMG-SNU Boramae Medical Center from January 2010 to December 2015. We only included patients younger than 30 years old who were evaluated in outpatient clinical settings. Patients diagnosed with epidermal cysts or those undergoing surgical excision were excluded. Age, sex, body sites of infection, medical histories, prescribed medications, and treatment outcomes were obtained from the medical records. The study protocol was approved by the Institutional Review Board of the SMG-SNU Boramae Medical Center (IRB no. 16-2016-48). The requirement of informed consent was waived by the Institutional Review Board.
The diagnosis of skin infections was further classified into primary and secondary infections. The primary infection was defined as a skin infection arising in the normal skin. It included impetigo, folliculitis, abscess, furuncle, carbuncle, cellulitis, and others. Secondary infection was defined as a skin infection that occurred at the site of underlying skin diseases, the diagnoses of which were obtained from information in the medical record. Underlying skin diseases included eczema, trauma, and other infections. The patients placed in the responder group showed resolution of infection after the initial treatment and subsequent treatment was thus terminated. Otherwise the patient was categorized into the non-responder group; in this group, there was little improvement after the initial treatment and the treatment had to be changed.
Skin swab cultures (sterile transport swab on Stuart agar gel medium, Copan Venturi Transystem®; Copan, Murrieta, CA, USA) were performed at suspected sites of skin infection and then inoculated on blood agar plates followed by McConkey agar plates. They were subsequently incubated in a carbon dioxide (CO2) incubator set at 35℃ and 5% CO2 for 1 day (Thermo Scientific Inc., Waltham, MA, USA). Gram staining was performed in the presence of colonies on inoculated culture medium. Gram-positive bacteria were judged to be S. aureus when their catalase and coagulase tests were positive, and CoNS was determined according to negative catalase and coagulase results. Subsequently, the specimens were inoculated on Mueller-Hinton agar and tested for susceptibility to antibiotics by disk diffusion method.
For other species, automation equipment, including VITEK 2 (bioMerieux Inc., Hazelwood, MO, USA) and MicroScan (Beckman Coulter Inc., Brea, CA, USA), was used for microbial identification and antibiotic susceptibility tests.
We used the chi-square test for categorical data and the independent t-test for continuous data to assess differences between the responder and the non-responder groups. Microbiological variables (bacterial species, methicillin susceptibility, resistance to a number of antibiotics, and culture amount) were all included in a multivariable logistic regression model to adjust for confounders. In the case of clinical variables (e.g., age, gender, diagnosis, affected location, treatment), only variables showing a univariable association with treatment response (p<0.20) were included in a multivariable logistic regression model. IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used for all analyses and p-values of less than 0.05 were considered statistically significant.
We identified 197 patients (108 males and 89 females) who underwent bacterial culture of skin lesions at the outpatient clinic. The mean age was 13.9±9.8 years. Secondary bacterial infection was more prevalent than primary infection in this population: 116 out of 197 patients (58.9%) showed bacterial infection from pre-existing skin lesions. The most common pre-existing dermatosis was atopic eczema. The most frequently involved site was the lower limbs (68.5%), followed by the face, upper limbs, and trunk (Table 1). For treatment, cephradine was the most frequently used systemic antibiotic agent (n=156) and mupirocin (n=89) was the most commonly used topical antibiotic agent.
Bacteria were isolated in 166 samples from 155 patients. The most frequently detected bacterial species were S. aureus (66.3%) and CoNS (15.7%). Of 155 culture-positive patients, most isolates were single pathogens (92.9%) and only 11 patients (7.1%) showed mixed pathogens.
Among 166 isolated bacteria samples, 160 samples had Gram-positive bacteria (96.4%) and only 6 samples had Gram-negative bacteria (3.6%). Among 160 samples isolating Gram-positive bacteria, penicillin resistance was found in 124 samples (77.5%) and methicillin-resistant species were found in 45 (28.1%). All methicillin-resistant species were either S. aureus or CoNS. In the case of S.aureus, 36.4% revealed methicillin resistance and 19.2% of CoNS were methicillin resistant. Of 166 culture-positive samples, 53 showed antibiotic resistance to more than 3 antibiotics (31.9%). Doxycycline, minocycline, clindamycin, TMP-SMX, and linezolid are known as the existing MRSA oral treatment agents8. Some species were also partially resistant to tetracycline (n=20, 12.5%) or clindamycin (n=41, 25.6%). Erythromycin-inducible, clindamycin-resistant S. aureus was also observed in 7 patients (6.4%).
The bacteriological characteristics according to the diagnosis were also assessed. Compared to rates of secondary infection with S. aureus, the proportion of methicillin-resistant species in primary infection was increased (50% vs. 26.6%, p=0.012). Additionally, the number of resistant antibiotics was increased compared to that of secondary infection, showing a statistically significant difference (p=0.022).
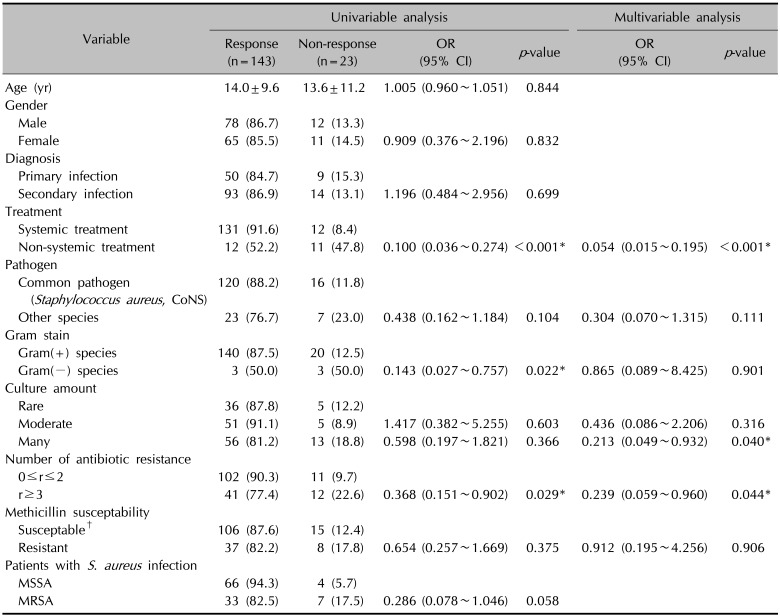
Among 197 patients, 170 patients (86.3%) showed clinical improvement after initial treatment (Table 1). As shown in Table 2, the univariable logistic regression analysis of 166 culture-positive samples revealed that Gram-negative species (p=0.022), resistance to 3 or more antibiotics (p=0.029), and non-systemic antibiotics use (p<0.001) were significantly associated with lower treatment response. There were no significant differences in treatment response according to age, sex, infection site, and diagnosis. Methicillin resistance was not associated with lower treatment response (p=0.375). In non-responders, azithromycin, ciprofloxacin, and moxifloxacin were used in the secondary treatment after failure of the initial treatment, but there was a limit to the analysis of the treatment response due to the small number of patients (Table 2).
In the non-responder group, the proportion of S. aureus was 18.5%, slightly higher compared to 12.4% in the responder group, and the proportion of MRSA and MRCoNS increased to 24.1% and 3.4%, respectively, but did not show a statistically significant difference. Regarding S. aureus, MRSA tended to have a lower response to treatment than methicillin-susceptible S. aureus (MSSA) (82.5% vs. 94.3%, respectively) but the difference was not statistically significant (p=0.058).
In the multivariable logistic regression analysis, resistance to 3 or more antibiotics (p=0.044) and non-systemic antibiotic use (p<0.001) remained significantly associated with lower treatment response, whereas the association with the type of Gram stain for a species was no longer significant. Instead, culture amounts quantified as “many” showed a significant association with lower treatment response compared to culture amount described as “rare,” when adjusting for covariates (p=0.040). Methicillin resistance was not associated with lower treatment response both in univariable and multivariable analyses.
In this retrospective analysis, we examined microbiological characteristics of skin infections and analyzed clinical and microbial factors in an effort to predict responses to treatment. These analyses targeted children and young adults without systemic underlying diseases in an outpatient setting. In this population, the response to initial treatment was high and even MRSA responded well to empirical antibiotic treatment, including use of first-generation cephalosporins. We also found that the number of antibiotic-resistant bacteria, the colony count, and the treatment regimen were associated with responsiveness.
Previous articles reported the results of culture tests on infected skin lesions in patients in the intensive care unit or emergency department910. However, the patient population in this study was different from that described in previous reports. This study included outpatient pediatric patients and young adults less than 30 years old without underlying systemic disorders. These patients commonly have secondary superficial bacterial infections, and less commonly have more deep-seated infections such as abscesses and cellulitis. They are commonly seen in outpatient dermatology clinics11. In addition, patients admitted to a secondary care hospital are considered to have community-acquired rather than hospital-acquired infections.
In the present study, the overall treatment response was 86.3%. An explanation for this high response was that empirical antibiotics mostly targeted Gram-positive bacteria, which accounted for 96.4% of isolates in this study.
We found that systemic antibiotics were significantly more effective than topical antibiotics for skin infections. Since patients with mild and limited localized infections were more likely to choose topical treatment, oral antibiotics are more likely to be effective than topical antibiotics under the same disease severity conditions. In some cases, contact dermatitis due to topical antibiotic application itself may be a concern, as in atopic disease12. In contrast, previous studies comparing the efficacy of topical and systemic therapies reported that the number of resistant pathogens increased with the use of systemic antibiotics13. Thus, oral antibiotics are recommended if skin infection is strongly suspected.
Systemic antibiotics may achieve superiority by preventing deterioration of a skin lesion through early intensive treatment, and by helping restore the skin barrier1415.
In this study, MRSA accounted for 36.4% of total S. aureus isolates, with infection rates increasing over time, compared with 9.8% in outpatients in 2006 and 16.6% in suspected infectious disease cases16. Consistent with other studies171819, S. aureus was the most frequently detected pathogen, and the vast majority of isolated S. aureus was resistant to penicillin, with intermediate or complete resistance to erythromycin, clindamycin, and gentamycin, in addition to oxacillin. Clindamycin is also considered a second-line drug in severe MRSA infections20, and can exhibit inducible resistance due to erythromycin as well as clindamycin alone21.
Among those with S. aureus skin infections, the proportion of MRSA was significantly higher in primary infection than in secondary infection. This could be because an infection in an intact skin barrier may have greater virulence than an infection in an already damaged skin barrier. It is also possible that the nature of susceptibility to antibiotics may be different2223.
Not only was S. aureus detected, but CoNS was also detected in 15.7% of cases in this study. CoNS, a species commonly present in the normal skin flora, has shown pathogenicity in catheter-related infections or immunosuppressed patients2425; however, even in immunocompetent patients, CoNS may become virulent if it enters the skin surface in the presence of a damaged skin barrier. The rate of resistance to various antibiotics is also increasing26. In fact, this study showed that the rate of MRCoNS is about 2.4%.
In the present study, the treatment response of MRSA to empirical antibiotic use was more than 80%. It has been reported that therapeutic response is obtained when appropriate drainage is combined with empirical systemic antibiotics, even without the use of intravenous agents such as vancomycin, which is currently recommended2. In addition, satisfactory therapeutic responses were obtained without the use of clindamycin or TMP-SMX, the agents recommended for outpatient treatment of MRSA in simple abscesses1. It is known that most community-associated MRSA isolates have Panton-Valentine leukocidin, which confers greater virulence than is seen with hospital-associated MRSA or MSSA2. Nevertheless, the results of this study confirm that skin infection in immunocompetent hosts can be treated with conventional dressings and oral systemic antibiotics such as cephalosporins310.
However, we also observed some trends toward antibiotic resistance, especially among patients with S. aureus infections. The number of antibiotics to which S. aureus showed resistance was associated with poorer treatment responses. Resistance to three or more antibiotics was shown by 31.9% of patients, and the response to treatment was somewhat poorer than in those with resistance to two or less. Furthermore, the treatment response was somewhat poorer in MRSA-infected patients than in MSSA-infected patients, even though there was no statistically significant difference in treatment response.
The results of this study do not apply to patients with hospital-associated MRSA or systemic infection. In addition, this study was performed with retrospective chart review and only patients who had swab cultures were included; the study was therefore limited to outpatients with infectious skin lesions. However, swab culture is generally performed in patients with more severe skin lesions; therefore, the bacteriological characteristics and therapeutic responses can be applied to superficial skin infections in the outpatient setting.
Despite limitations, the present study showed that among immunocompetent children and young adults, the response rate to empirical treatment with systemic antibiotics targeting Gram-positive bacteria was high. Moreover, first-generation cephalosporins could still be used in a first-line empirical regimen for bacterial skin infections, especially in cases of secondary infection treated in the outpatient setting, regardless of methicillin resistance. Because the treatment success rate with topical agents is significantly less, these should be avoided if a lesion is not mild or focal. Antibiotic-resistance testing is recommended. However, if testing is not feasible, the absence of resistance to TMP-SMX in this study should be noted. Therefore, as in previous studies, this drug may be suitable as a second-line option when first-generation cephalosporin treatment fails. However, because serious side effects such as rashes, allergic reactions, or bone marrow suppression are not rare, TMP-SMX is still recommended as a second-line treatment.
References
1. Daum RS, Miller LG, Immergluck L, Fritz S, Creech CB, Young D, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017; 376:2545–2555. PMID: 28657870.

2. Ellis MW, Lewis JS 2nd. Treatment approaches for community-acquired methicillin-resistant Staphylococcus aureus infections. Curr Opin Infect Dis. 2005; 18:496–501. PMID: 16258322.

3. Odell CA. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections. Curr Opin Pediatr. 2010; 22:273–277. PMID: 20386450.

4. Chen AE, Carroll KC, Diener-West M, Ross T, Ordun J, Goldstein MA, et al. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatrics. 2011; 127:e573–e580. PMID: 21339275.

5. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014; 59:147–159. PMID: 24947530.
6. Leung DYM, Eichenfield LF, Boguniewicz M. Atopic dermatitis (atopic eczema). In : Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill;2012. p. 165–182.
7. Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010; 125:4–13. quiz 14-15. PMID: 20109729.

8. Lowy FD. Staphylococcal infections. In : Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 19th ed. New York: McGraw-Hill;2014. p. 954–962.
9. Lepainteur M, Royer G, Bourrel AS, Romain O, Duport C, Doucet-Populaire F, et al. Prevalence of resistance to antiseptics and mupirocin among invasive coagulase-negative staphylococci from very preterm neonates in NICU: the creeping threat? J Hosp Infect. 2013; 83:333–336. PMID: 23414707.

10. Walraven CJ, Lingenfelter E, Rollo J, Madsen T, Alexander DP. Diagnostic and therapeutic evaluation of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections in the emergency department. J Emerg Med. 2012; 42:392–399. PMID: 21524884.

11. Lee HJ, Choi SI, Ahn SK. A statistical study of dermatoses in Wonju (2005~2009). Korean J Dermatol. 2010; 48:460–467.
12. Gehrig KA, Warshaw EM. Allergic contact dermatitis to topical antibiotics: epidemiology, responsible allergens, and management. J Am Acad Dermatol. 2008; 58:1–21. PMID: 18158924.

13. Parish LC, Jorizzo JL, Breton JJ, Hirman JW, Scangarella NE, Shawar RM, et al. Topical retapamulin ointment (1%, wt/wt) twice daily for 5 days versus oral cephalexin twice daily for 10 days in the treatment of secondarily infected dermatitis: results of a randomized controlled trial. J Am Acad Dermatol. 2006; 55:1003–1013. PMID: 17097398.

14. Gulliford MC, Moore MV, Little P, Hay AD, Fox R, Prevost AT, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ. 2016; 354:i3410. PMID: 27378578.

15. Napolitano LM. Early appropriate parenteral antimicrobial treatment of complicated skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. Surg Infect (Larchmt). 2008; 9(Suppl 1):s17–s27. PMID: 18844471.
16. Kim MS, Chung BS, Choi KC. A study of antibiotic susceptibility of Staphylococcus aureus in bacterial skin infections. Korean J Dermatol. 2006; 44:805–810.
17. Jung MY, Chung JY, Lee HY, Park J, Lee DY, Yang JM. Antibiotic susceptibility of Staphylococcus aureus in atopic dermatitis: current prevalence of methicillin-resistant Staphylococcus aureus in Korea and treatment strategies. Ann Dermatol. 2015; 27:398–403. PMID: 26273155.
18. Kim WJ, Lee KR, Lee SE, Lee HJ, Yoon MS. Isolation of the causative microorganism and antimicrobial susceptibility of impetigo. Korean J Dermatol. 2012; 50:788–794.
19. Park JH, Byun JY, Lee DY, Lee JH, Yang JM, Lee ES. Trends of the bacterial skin infections of dermatology outpatients in 1996, 2001 and 2006. Korean J Dermatol. 2009; 47:690–695.
20. Prabhu K, Rao S, Rao V. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. J Lab Physicians. 2011; 3:25–27. PMID: 21701659.

21. Woods CR. Macrolide-inducible resistance to clindamycin and the D-test. Pediatr Infect Dis J. 2009; 28:1115–1118. PMID: 19935273.

22. Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol. 2016; 51:329–337. PMID: 27377298.

23. Choi JH, Seo HS, Lim SY, Park K. Cutaneous immune defenses against Staphylococcus aureus infections. J Lifestyle Med. 2014; 4:39–46. PMID: 26064853.
24. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999; 50:223–236. PMID: 10073274.
25. Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014; 27:870–926. PMID: 25278577.

26. Archer GL. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev Infect Dis. 1991; 13(Suppl 10):S805–S809. PMID: 1754789.

Table 1
Patient demographics and clinical characteristics (n=197)
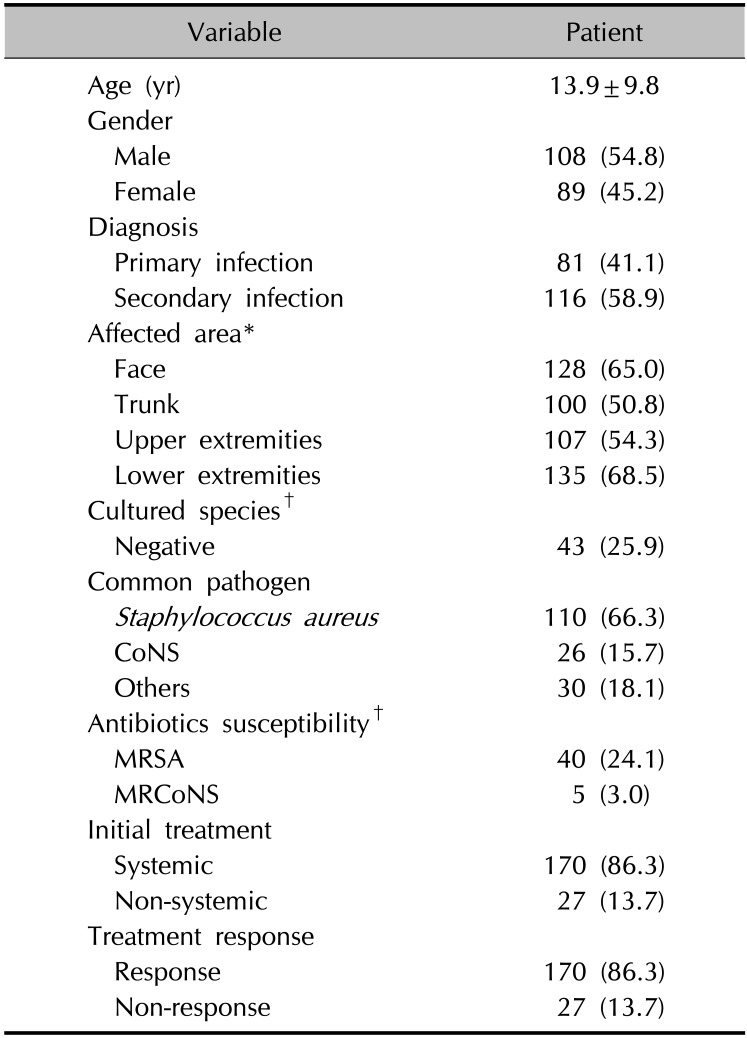
Table 2
Univariable and multivariable analysis of treatment response for culture-positive samples (n=166)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download