Abstract
Background
Topical tacrolimus is an effective anti-inflammatory therapy for acute and chronic states of atopic dermatitis (AD) in both adults and children. Topical tacrolimus has particular use at sensitive areas such as the face, anogenitals, and skin folds of neck and extremities. However, many AD patients also experience aggravated symptoms on trunk.
Objective
The aim of this study was to investigate the efficacy and safety of topical tacrolimus for AD patients with truncal lesions.
Methods
AD patients with truncal lesions who were aged ≥2 years were recruited from 20 centres in Korea. They received treatment with topical tacrolimus ointment twice daily during 4 weeks. The primary end point was change of the local eczema area and severity index (EASI) of the trunk from baseline to day 28. The secondary end points were changes in the patient global assessment (PGA) score and itch visual analogue scale (VAS) score of the trunk between baseline and day 28.
Results
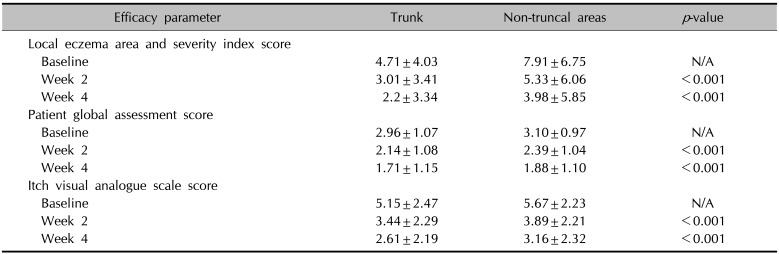
Two hundred and ninety-one patients were recruited, and 176 patients completed the full 4-week treatment course. By the end of the treatment, the mean local EASI of the trunk (2.2±4.71) was significantly decreased from that at baseline (4.71±4.03, p<0.001). PGA (1.71±1.15) and itch VAS score of the trunk (2.61±2.19) on day 28 were also profoundly decreased compared with the baseline (2.96±1.07 and 5.15±2.47, respectively). No serious adverse events were observed during the study period.
Atopic dermatitis (AD) is a chronically relapsing, common inflammatory skin disease1. Topical corticosteroids are the mainstay treatment of AD. However, they carry the risk of local side effects such as the development of skin atrophy, telangiectasia, acne, and striae, and systemic side effects that result from the suppression of the hypothalamic-pituitary-adrenal axis, especially in cases with long-term use. Indeed, topical mid- to high-potency corticosteroids are difficult to use regularly on the face and intertriginous areas because of the local side effects2. For the above-mentioned reasons, topical tacrolimus has been particularly used at sensitive areas such as the face, anogenitals, and skin folds of neck and extremities, where the risk of the adverse effects of topical corticosteroids is higher3. Although many AD patients also experience aggravated symptoms in the truncal area4, only few studies have assessed the efficacy and safety of tacrolimus ointment for the treatment of AD on the truncal area.
AD patients with truncal lesions who were aged ≥2 years were recruited from 20 centres in Korea. AD was diagnosed in accordance with the criteria of Hanifin and Rajka5. Patients with truncal lesions affecting >1% of body surface area were selected. The exclusion criteria applied at recruitment were history of topical tacrolimus therapy within the previous 4 weeks, active infection, malignancy or uncontrolled chronic illness, pregnancy or nursing state, immunosuppressed state, any serious skin disorder, and presence of contraindication to topical tacrolimus (e.g., skin ulcer, hypersensitivity to tacrolimus). Written informed consent was obtained from the participant, and if age is under 18, from the parent or legal guardian.
The present study was approved by the institutional review board of Pusan National University Yangsan Hospital (IRB no. 04-2015-006), The Catholic University of Korea, Incheon St. Mary's Hospital (IRB no. OIRB-00243-007), Soonchunhyang University Bucheon Hospital (IRB no. SCHBC 2015-03-003), Wonju Severance Christian Hospital (IRB no. CR115003-005), Korea University Ansan Hospital (IRB no. AS14189-002), Kyung Hee University Hospital at Gangdong (IRB no. KHNMC 2015-02-030-001), Konkuk University Medical Center (IRB no. KUH1120064), Chungnam National University Hospital (IRB no. CNUH 2015-06-039-003), The Catholic University of Korea, St. Vincent's Hospital (IRB no. VIRB-00124-001), Samsung Changwon Hospital (IRB no. 2015-SCMC-027-00), Chosun University Hospital (IRB no. CHOSUN 2015-03-011), Asan Medical Center (IRB no. 2015-0662), Dongguk University Ilsan Hospital (IRB no. 2015-32), Severance Hospital (IRB no. 4-2015-0037), Gachon University Gil Medical Center (IRB no. GCIRB2015-110), The Catholic University of Korea, Seoul St. Mary's Hospital (IRB no. KC15MIMI0221), Kyungpook National University Hospital (IRB no. KNUH 2015-03-038-002), Chonbuk National University Hospital (IRB no. CUH 2015-03-030-001), Ajou University Hospital (IRB no. AJIRB-MED-OBS-15-046), Hallym University Kangnam Sacred Heart Hospital (IRB no. 2015-02-21), Korea.
Tacrolimus ointment 0.1% (for patients aged ≥19 years) and 0.03% (for patients aged <19 years) were applied twice daily to all areas of active disease. The amounts of topical tacrolimus ointment applied to AD lesions were measured in fingertip units67. The treatment was continued for 4 weeks, regardless of whether complete clearance in all baseline treatment areas had been achieved. The patients were prohibited to receive either systemic or topical corticosteroids, or immunosuppressive drugs or phototherapy (ultraviolet [UV] A, UVB) or antibiotics, except in the case of controlling infected AD with topical antibiotics, herbal remedy, γ-linolenic acid, and other drugs in the investigation during the study. The patients were permitted to take oral antihistamines and to use non-medicated emollients as needed throughout the study.
The patients were evaluated at baseline and at weeks 2 and 4 after treatment. The primary efficacy end point was the change from baseline in local eczema area and severity index (EASI) score of the trunk on day 2889. The secondary end points were the changes patient global assessment (PGA) and itch visual analogue scale (VAS) score of trunk from baseline to day 28. In addition, changes in local EASI, PGA and itch VAS score of the trunk during the study period were compared with those of non-truncal areas (head, neck and extremities). All adverse events during the investigation period were recorded.
Changes in overall clinical status were rated in accordance with the patient's global assessment scores based on a 5-point scale defined by a score of 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), 4 (severe), or 5 (very severe). The patients were also asked to assess pruritus by using an itch VAS score, where 0 indicated no itching sense and 10 indicated the worst itch imaginable.
Local EASI, PGA and itch VAS scores of the trunk and non-truncal areas were evaluated simultaneously in the study. Local EASI score of the each body region in this study was defined as multiplying the sum of the severity scores for each symptom by the area score and then multiplying the result by a constant weighted value assigned to that body region, so the sum of each local EASI score of trunk and non-truncal areas was equal to patient's EASI score. Local PGA score and itch VAS score of the trunk were also obtained from the patients' subjective assessments, which were localized to the trunk. All adverse events, including burning sensation, itching sensation, erythema, aggravation of skin lesion, skin infection, and other localized and systemic adverse events during the investigation period were recorded.
All statistical analyses were performed by using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). We used the paired Wilcoxon test for analysis of primary and secondary end points, and Mann-Whitney test was used to compare changing in local EASI, PGA and itch VAS score of the trunk with those of non-truncal areas. The p-values of <0.05 were considered statistically significant.
For this study, 291 patients were recruited and 176 patients (99 males and 77 females; mean age, 18.3 years; range, 2~59 years) completed the full 4-week treatment course. Eighty-three patients were aged ≥19 years, while 93 patients were aged <19 years. The mean baseline EASI scores of all the patients who completed the study, the patients aged ≥19 years, and the patients aged <19 years were 12.62±10.03, 14.86±11.72, and 10.62±7.77, respectively. During the clinical trials, 115 patients did not complete the study for several reasons. The most common cause of discontinuation was simple follow-up loss (n=103, 89.6%), followed by voluntary withdrawal (n=5, 4.3%), lack of efficacy (n=4, 3.5%), adverse events (n=2, 1.7%), and aggravated AD symptoms (n=1, 0.9%) (Table 1).
Statistical clinical improvements in both the trunk and non-truncal areas were observed over the treatment period. By the end of the treatment period, the mean EASI score of the whole body (6.19±8.91) was statistically improved from the baseline (12.62±10.03), while the mean local EASI score of the trunk (2.2±3.34) and non-truncal areas (3.98±5.85) was also significantly decreased from those at baseline (4.71±4.03 and 7.91±6.75, respectively). The mean PGA score of the trunk (1.71±1.15) and non-truncal areas (1.88±1.10) at week 4 were notably improved from those at baseline (2.96±1.07 and 3.10±0.97, respectively). The mean itch VAS score of the trunk (2.61±2.19) and non-truncal areas (3.16±2.32) were also profoundly decreased from those at baseline (5.15±2.47 and 5.67±2.23, respectively) (Table 2). In addition, the rate of improvement in mean local EASI, PGA and itch VAS score of the trunk from those at baseline were statistically higher than those of non-truncal areas (53.3% vs. 49.7%, 42.2% vs. 39.4% and 49.3% vs. 44.3%, respectively (Fig. 1).
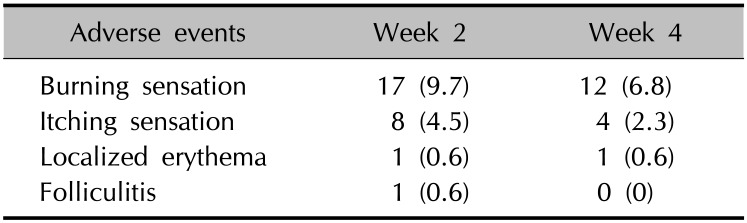
Adverse events were monitored by the investigators at each visit by history taking and physical examination. No serious adverse events were observed, except local reactions, including burning sensation, itching sensation, localized erythema, and folliculitis (Table 3). In the group of patients who completed the 4-week study course, a burning sensation was observed in 9.7% (n=17) of the patients at week 2 and in 6.8% (n=12) at week 4. Itching sensation was observed in 4.5% (n=8) of the patients at week 2 and in 2.3% (n=4) at week 4. Localized erythema and folliculitis were observed only in one patient, respectively. As the results, incidence of burning and itching sensation of topical tacrolimus tended to decrease as the study progressed. Only two (0.7%) of the 291 patients were dropped out of the study because of adverse events due to topical tacrolimus. One had both burning and itching sensations, and the other had only itching sensation. No systemic symptoms were observed during the 4-week study period.
Topical tacrolimus is an effective anti-inflammatory therapy for acute and chronic states of AD in both adults and children1234. It is usually applied to sensitive areas such as the face, anogenitals, and skin folds of neck and extremities, where the adverse risk from topical corticosteroids is higher3. Several reports support its usefulness as steroid-sparing agent in the case of recalcitrant AD to topical corticosteroids, corticosteroid-induced skin atrophy, and long-term use of uninterrupted topical corticosteroids101112.
Many AD patients experience aggravated symptoms in the trunk and other body parts such as face and flexural areas, and physicians are familiar with using topical corticosteroids to the truncal area413. The usefulness of topical tacrolimus for the truncal area in AD patients tends to be overlooked for some reasons as follows. First, the large body surface area over the trunk can be a burden to physicians because of systemic absorption of tacrolimus. Second, AD patients' adherence to treatment for the truncal area could be lower than that for other body parts because of reasons such as large surface of the trunk itself and difficulties of applying14. The tacrolimus ointment also sometimes could feel unpleasant because it easily stains clothing and interferes with daily activities15. In addition, lack of clinical evidence for the efficacy and safety of topical tacrolimus to the trunk could make physicians hesitate to use topical tacrolimus. Many studies reported the efficacy and safety of topical tacrolimus in the treatment of AD, but no report has focused on the efficacy and safety of topical tacrolimus to the trunk itself, and topical steroids are still the mainstay of AD treatment, especially to the trunk1234.
In our study, we focused on the efficacy of topical tacrolimus to the trunk comparing with non-truncal areas. Local EASI, PGA, and itch VAS score examined in this study demonstrated that topical tacrolimus is not only effective to the facial or flexural area but also the trunk. The rate of improvement in all the parameters showed even better on the trunk than on the non-truncal areas. Overestimation of the efficacy based on the local PGA and itch VAS scores could be possible because both PGA and itch VAS scores are subjective symptoms of patients and, the trunk is a less exposed area than the other body parts such as the face and flexural area. However, in our study, the rate of improvement in local EASI score was also better on the trunk comparing non-truncal areas.
Although any adherence profile was not imposed on patients to complete the study, we obtained enough results to prove the efficacy of topical tacrolimus to the trunk. It can be meaningful for physicians who worry about low adherence of topical tacrolimus to the trunk in AD patients141516.
Treatment with topical tacrolimus was generally well tolerated. Only two of the 291 patients (0.7%) discontinued the study because of adverse events. The adverse events were limited to local reactions, including burning sensation, followed by itching sensation, erythema, and folliculitis. Leung et al.2 stated that topical tacrolimus applied on up to 100% of the body surface in adults and children have shown no significant systemic adverse effects. Cury Martins et al.17 also concluded the systemic safety of topical tacrolimus in their review article with 5,885 participants, as systemic absorption was rarely detectable, only in low levels, and this decreased with time. Recently, the importance of proactive treatment of AD has been highlighted1819. However, most patients might hesitate to use topical corticosteroids to the trunk as a proactive treatment because of several severe side effects after systemic corticosteroids absorption, such as Cushing syndrome due to the large surface area of the trunk2021. Charman et al.22 reported 72.5% of adult AD patients or parents children with AD had admitted to being worried about using topical corticosteroids. Based on our results, topical tacrolimus can be a good option for proactive treatment in AD patients with corticosteroids phobia23.
The limitations of our research are as follows: Our research was an observational study, so we could not compare the effectiveness of topical tacrolimus with other treatments for the truncal area in AD patients.
In summary, this study showed that topical tacrolimus seems to be effective and safe for treating truncal AD. We believe our study could be informative for many physicians who hesitate to use topical tacrolimus on the trunk because of the lack of clinical evidence for efficacy and safety.
ACKNOWLEDGMENT
The present study was conducted with financial support from Astellas Pharmaceutical Company, which produces a topical tacrolimus ointment (Protopic).
References
1. Won CH, Seo PG, Park YM, Yang JM, Lee KH, Sung KJ, et al. A multicenter trial of the efficacy and safety of 0.03% tacrolimus ointment for atopic dermatitis in Korea. J Dermatolog Treat. 2004; 15:30–34. PMID: 14754647.

2. Leung DY, Nicklas RA, Li JT, Bernstein IL, Blessing-Moore J, Boguniewicz M, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004; 93(3 Suppl 2):S1–S21.
3. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014; 71:116–132. PMID: 24813302.
4. Reitamo S, Van Leent EJ, Ho V, Harper J, Ruzicka T, Kalimo K, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone acetate ointment in children with atopic dermatitis. J Allergy Clin Immunol. 2002; 109:539–546. PMID: 11898004.

5. Hanifin JM, Rajka R. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
6. Long CC, Finlay AY, Averill RW. The rule of hand: 4 hand areas = 2 FTU = 1 g. Arch Dermatol. 1992; 128:1129–1130. PMID: 1497374.

7. Kalavala M, Mills CM, Long CC, Finlay AY. The fingertip unit: a practical guide to topical therapy in children. J Dermatolog Treat. 2007; 18:319–320. PMID: 17852630.

8. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001; 10:11–18.
9. Tofte S, Graeber M, Cherill R, Omoto M, Thurston M, Hanifin J, et al. Eczema area and severity index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol. 1998; 11(Suppl 2):S197.

10. Murrell DF, Calvieri S, Ortonne JP, Ho VC, Weise-Riccardi S, Barbier N, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol. 2007; 157:954–959. PMID: 17935515.

11. Kawakami T, Soma Y, Morita E, Koro O, Yamamoto S, Nakamura K, et al. Safe and effective treatment of refractory facial lesions in atopic dermatitis using topical tacrolimus following corticosteroid discontinuation. Dermatology. 2001; 203:32–37. PMID: 11549797.

12. Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. Br J Dermatol. 2009; 160:415–422. PMID: 19067708.

13. Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005; 152:1282–1289. PMID: 15948994.

14. Moftah NH, Ibrahim SM, Wahba NH. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: a comparative randomized split body study. Arch Dermatol Res. 2016; 308:263–268. PMID: 26993345.

15. Devaux S, Castela A, Archier E, Gallini A, Joly P, Misery L, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012; 26(Suppl 3):61–67. PMID: 22512682.

16. Jin H, Kim JM, Kim GW, Kim HS, Ko HC, Kim MB, et al. Inappropriate amounts of topical tacrolimus applied on Korean patients with eczema. J Dermatolog Treat. 2017; 28:327–331. PMID: 27588441.

17. Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015; (7):CD009864. PMID: 26132597.

18. Wollenberg A, Reitamo S, Atzori F, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008; 63:742–750.

19. Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011; 164:415–428. PMID: 20819086.

20. Tiwari A, Goel M, Pal P, Gohiya P. Topical-steroid-induced iatrogenic Cushing syndrome in the pediatric age group: a rare case report. Indian J Endocrinol Metab. 2013; 17(Suppl 1):S257–S258. PMID: 24251179.
21. Gen R, Akbay E, Sezer K. Cushing syndrome caused by topical corticosteroid: a case report. Am J Med Sci. 2007; 333:173–174. PMID: 17496736.

22. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000; 142:931–936. PMID: 10809850.

23. Lee JY, Her Y, Kim CW, Kim SS. Topical corticosteroid phobia among parents of children with atopic eczema in Korea. Ann Dermatol. 2015; 27:499–506. PMID: 26512163.

Fig. 1
The rate of improvements in local eczema area and severity index (EASI), patient global assessment (PGA) and itch visual analogue scale (VAS) score of trunk from baseline to week 4 (53.3%, 42.2%, 49.3%, respectively) was significantly higher than those of the non-truncal areas (49.7%, 39.4%, 44.3%, respectively). *The rate of improvements in each parameter in comparison with week 4 and baseline. †,‡Statistically significant difference between trunk and non-truncal areas, †p<0.001, ‡p<0.05 (Mann-Whitney test).
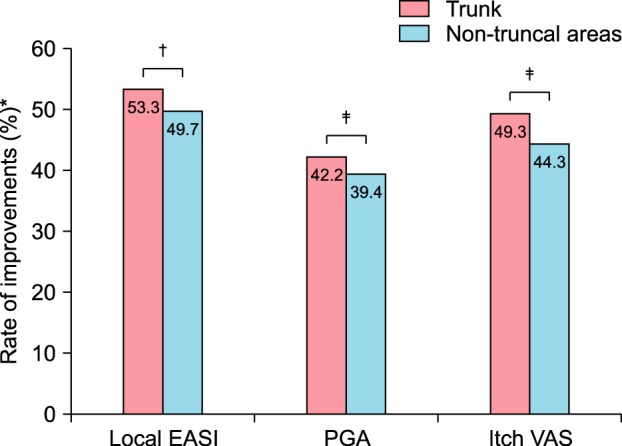
Table 1
Demographics of the patients and causes of drop out (n=291)
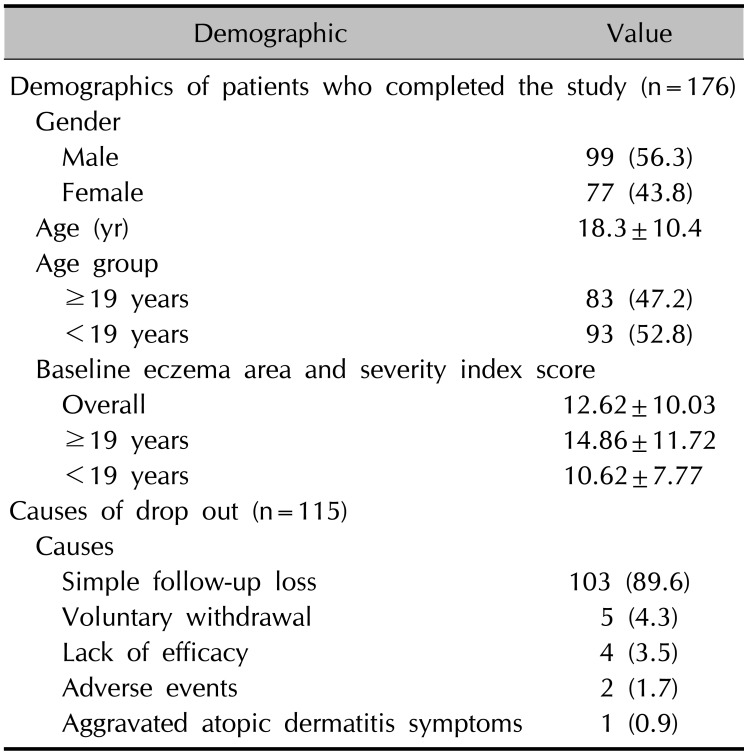
Table 2
Changing in efficacy parameters on trunk and non-truncal areas at baseline, week 2, and week 4




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download