Abstract
Background
Increasing evidence suggests a pivotal role for neuronal inflammation in response to replicating varicella zoster virus in the development of postherpetic neuralgia (PHN).
Objective
In this study, we investigated the value of serum levels of various inflammatory markers in acute herpes zoster (HZ) as predictors for the development of PHN.
Methods
A total of 116 patients with acute HZ were enrolled in this study. We measured scores on the pain visual analogue scale (VAS) at baseline and at 1, 3, and 6 months after diagnosis of HZ. We defined PHN as pain greater than 1 on the VAS lasting for more than 6 months. Serum samples for laboratory assay, including complete blood count were obtained at the initial visit. Correlations between the levels of each inflammatory marker and the development of PHN were evaluated.
Results
Levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), lymphocyte count, and albumin showed significant correlations with development of PHN in univariate analysis. Levels of ESR, CRP, and lymphocyte count also showed significant correlations in multivariate analysis. ESR level showed stronger correlations with development of PHN than levels of CRP and lymphocyte count.
Postherpetic neuralgia (PHN) is characterized by spontaneous pain, pain provoked by trivial stimuli, and altered sensations that accompany herpes zoster (HZ) and that may continue long after the characteristic rash of HZ has healed1. PHN is neuropathic and results from injury of the peripheral nerves and altered central nervous system signal processing2. These changes may be so complex that no single therapeutic approach will ameliorate all of the abnormalities. As a result, antiviral agents and active interventional pain management during the early period of acute HZ are recommended to prevent the development of PHN3. Various risk factors for development of PHN such as old age, female sex, presence of a prodrome, more severe rash, and more severe acute pain reflect different mechanisms leading to the development of PHN4. For the timely identification of patients with HZ who might benefit from preventive strategies, it is important to identify the factors that can best predict the development of PHN. However, prediction of development of PHN is currently limited to clinical factors. A few reports showed that objective assessment tools such as the varicella zoster virus (VZV) skin-test reaction and infrared thermography are useful as predictors of development of PHN in patients with acute HZ, but more objective markers that can be easily available for early prediction of development of PHN are needed56.
Recently, neuronal inflammation secondary to replicating VZV has been identified as a potential mediator in the development of PHN. Therefore, we investigated whether early serum levels of various inflammatory markers in acute HZ could be useful to predict PHN development.
This study was undertaken in the Department of Dermatology at Kyungpook National University Hospital. From June 2013 to March 2015, a total of 116 patients with acute HZ were enrolled. HZ was diagnosed based on clinical presentation. Inclusion criteria were patients with laboratory results obtained within several hours after diagnosis of HZ and with a follow-up pain visual analogue scale (VAS) score of at least 1, a month later. Exclusion criteria included a confirmed history or suspicion of a congenital immune disorder; any chronic renal, hepatic, and rheumatologic illness; an acute infection within the previous month; trauma and/or fracture within the previous 6 months; and current use of steroids, including inhalers or nonsteroidal anti-inflammatory drugs. The protocol for this study was approved by the institutional review board of the Kyungpook National University Hospital (KNUH 2016-04-001).
We measured pain VAS scores at baseline and at 1, 3, and 6 months after diagnosis of HZ. Serum samples for laboratory assays, including complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and albumin level were obtained at the initial visit. The patients were divided into two groups, based on the presence or absence of pain at each month and the data in each group were statistically compared. We defined PHN as score of greater than 1 on the pain VAS scale having lasted for more than 6 months after onset of acute HZ. Pain at 6 months was criterion for taking statistics on this study.
We first evaluated correlations between levels of each serum inflammatory marker and development of PHN in univariate analysis. Already known predictive factors such as age, sex, and initial pain VAS score were also included as variables in the analysis. Then, we performed multivariate logistic regression analysis to identify which inflammatory markers independently correlated with development of PHN. To assess the actual influences of each serum inflammatory marker, clinical factors such as age, sex, and initial pain were excluded in multivariate analysis. Subsequently, cut-off values of the correlated inflammatory markers were established. Models composed of each inflammatory marker's cut-off value as well as age and initial pain VAS score were made for confirmation of each marker's influence on the prediction of development of PHN. The models' abilities were compared to each other using the area under the receiver operating characteristic (ROC) curves. Differences in area under the curve (AUC) between the reference model derived from only age and initial pain VAS score and a single factor added model were estimated. A p-value <0.05 was defined as statistically significant. All statistical analyses were performed using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA).
The characteristics of the 116 patients, including clinical information and mean initial laboratory results in each group at initial visit and at 1, 3, and 6 months are presented in Table 1. At the initial visit, the mean age of participants was 59.2 years (standard deviation [SD], 15.6); there were 42 male patients (36.2%) and 74 female patients (63.8%). Mean initial pain VAS score was 4.60 (SD, 2.30). The pain after diagnosis of HZ persisted for 1 month in 73 of 116 patients (62.9%), by 3 months in 42 of 100 patients (42.0%), and by 6 months in 20 of 93 patients (21.5%).
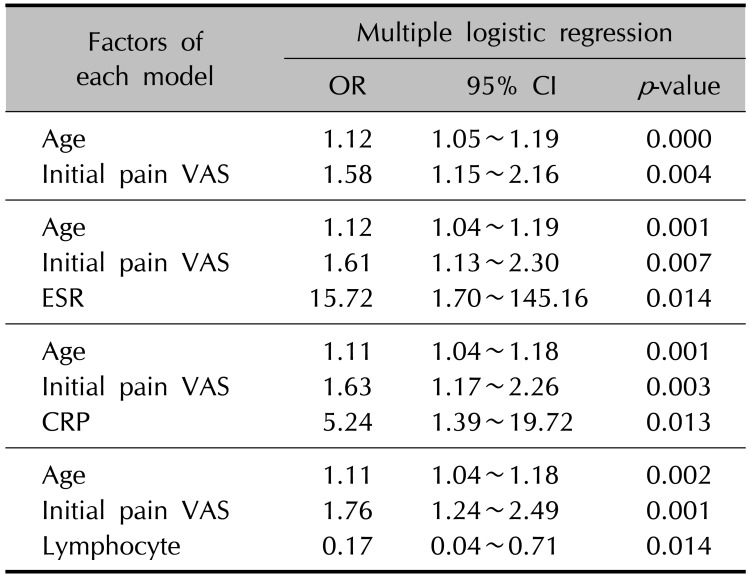
In univariate analysis, initial pain VAS score, levels of ESR, CRP, lymphocyte count, and albumin showed significant correlations with development of PHN at 6 months (p<0.05, Table 2). Other inflammatory markers, including white blood cell (WBC), red blood cell distribution width (RDW), platelet (PLT) count, neutrophil count, and neutrophil lymphocyte ratio (NLR) did not show significant correlations with development of PHN. There was no significant correlation between female sex and development of PHN (P>0.05, Table 2). Levels of ESR, CRP, and lymphocyte count showed significant correlations with development of PHN in a multivariate analysis conducted excluding clinical factors such as age, sex, and initial pain VAS score (p<0.05, Table 2). Cut-off values of statistically significant inflammatory markers for development of PHN were established and the results were 10.6 (p=0.040) in ESR, 0.34 (p=0.004) in CRP, and 1,297.2 (p=0.032) in lymphocyte count. Multivariate analysis in each model also showed significant correlations between each inflammatory marker's cut-off value and development of PHN (p<0.05, Table 3). Finally, we evaluated inflammatory markers that could improve the ability to predict PHN development in combination with age and initial pain VAS score, which are known to be the most important PHN predictors. The AUC increased significantly only when ESR values were combined with age and initial pain VAS scores (p<0.05, Fig. 1).
Although PHN is a common neuropathic complication of acute HZ that can reduce quality of life, the exact pathophysiology of PHN is not yet clearly understood7. It is known that pathologic interactions between afferent A beta- fibers with impaired function, axon reflexes with impaired responses, damaged C fibers, and a hyperreflexive spinal cord may play a role in pain associated with HZ as well as PHN8. Additionally, it has been reported that the immune system plays a crucial role in neuropathic pain, which includes interaction between inflammatory immune and immune-like glial cells, as well as inflammatory cytokines and chemokines9. This mechanism led to the emergence of the notion of neuropathic pain and suggested that the balance between pro- and anti-inflammatory cytokines determines whether neuropathic pain develops or not. However, it is not easy to predict the occurrence of neuropathic pain such as PHN. Several studies have indicated the significant contribution of inflammatory cells and their mediators, such as interleukin (IL)-6, to neuropathic pain1011. Although a previous study reported no significant differences in serum cytokine concentrations between HZ patients with and without PHN, a recent study showed that the levels of IL-6 in patients with PHN were higher than those in patients without PHN1213. Therefore, it was suggested that patients with nerve injury or an excessive inflammatory response to VZV may have a higher risk of development of PHN14. However, assessments of proinflammatory cytokines such as IL-6 are difficult to obtain in general practice. Therefore, there is a clinical need to identify more easily available inflammatory markers that can predict the development of PHN in patients with acute HZ. During the inflammatory reaction, several proinflammatory cytokines such as IL-1β, IL-6, IL-8, and tumor necrosis factor-α are produced, and these cytokines play an important role in the production of acute phase proteins15. That is, acute phase reactants reflect the presence and severity of inflammation. Importantly, CBC and levels of ESR, CRP are widely available, inexpensive, and routinely performed in general practice; moreover, they are accurate and standardized in many settings and can provide objective information to estimate patient prognosis. Therefore, we focused on those inflammatory markers in this study.
There have been a few reports on ESR and CRP as predictive factors for the development of PHN16. A previous study showed that ESR, CRP, and WBC count are not meaningful predictive factors for the development of PHN, but found that ESR was significantly higher in the 1-month PHN group in univariate analysis7. However, our study showed that levels of ESR, CRP, lymphocyte count, and albumin are significantly correlated with development of PHN at 6 months, and the possibilities of high levels of ESR, CRP, and lymphocyte count as predictors were confirmed in this study (Table 2). As expected, CBC, including WBC, RDW, PLT count, neutrophil count, and NLR were not predictors of PHN. However, our results differed from that of the previous study in that female sex, one of the already known predictive factors, was not a predictor of PHN in this study (Table 2). When we examined the actual influence of each inflammatory marker in combination with already known clinical factors such as age and initial pain VAS score, a model including ESR showed a better prediction power for development of PHN than models including CRP or lymphocyte count (Fig. 1). Levels of CRP and lymphocyte count did not show any additional considerable influence because clinical factors such as age and initial pain VAS score alone had sufficiently good predictive power.
PHN has been variably defined as any pain after acute infection or any pain at 1 month, 3 months, 4 months, or 6 months after rash onset417. In this study, we defined PHN as score greater than 1 on the pain VAS scale, lasting for more than 6 months after onset of acute HZ. This cut-off was chosen because we reasoned that if there was sustained pain for a long period of 6 months, it would be sufficient to meet any definition of PHN.
In conclusion, we suggest that high levels of ESR in acute HZ may be associated with the development of long-lasting PHN and that these inflammatory markers in combination with clinical factors such as age and initial pain VAS score will help to predict development of PHN more precisely. Therefore, we need to have more interest in the results of initial routine laboratory tests including these inflammatory markers as well as already known clinical factors in acute HZ patients. Furthermore, we suggest that anti- inflammatory treatment during the early phase of acute HZ is needed to prepare for the development of PHN, especially if the laboratory results show a high ESR. In such cases, patients will require regular long-term follow up for prevention and management of PHN.
This study provides insight into the role of inflammation in the development of PHN. We hope knowledge of these predictive factors may help researchers understand the pathogenesis of PHN and aid in development and evaluation of preventive interventions. A relatively small sample size and data from a single center are limitations of this study. Further investigations employing randomized clinical trials with a larger number of patients are required in the future.
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (NRF-2015R1D1A3A01016229).
References
1. Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996; 335:32–42. PMID: 8637540.
2. Wall PD. Neuropathic pain and injured nerve: central mechanisms. Br Med Bull. 1991; 47:631–643. PMID: 1794076.

3. Jang YH, Lee JS, Kim SL, Chi SG, Lee WJ, Lee SJ, et al. Do interventional pain management procedures during the acute phase of herpes zoster prevent postherpetic neuralgia in the elderly?: a meta-analysis of randomized controlled trials. Ann Dermatol. 2015; 27:771–774. PMID: 26719654.

4. Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004; 62:1545–1551. PMID: 15136679.

5. Ko EJ, No YA, Park KY, Li K, Seo SJ, Hong CK. The clinical significance of infrared thermography for the prediction of postherpetic neuralgia in acute herpes zoster patients. Skin Res Technol. 2016; 22:108–114. PMID: 26081167.

6. Imoto K, Okazaki A, Onishi F, Miyazaki Y, Okeda M, Yano S, et al. VZV skin-test reaction, but not antibody, is an important predictive factor for postherpetic neuralgia. J Dermatol Sci. 2015; 79:235–240. PMID: 26070505.

7. Kim YN, Kim DW, Kim ED. Efficacy of continuous epidural block in acute herpes zoster: Incidence and predictive factors of postherpetic neuralgia, a retrospective single-center study. Medicine (Baltimore). 2016; 95:e4577. PMID: 27512887.
8. Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000; 16(2 Suppl):S12–S20. PMID: 10870735.

9. Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010; 229:26–50. PMID: 20870295.

10. Kraychete DC, Sakata RK, Issy AM, Bacellar O, Jesus RS, Carvalho EM. Proinflammatory cytokines in patients with neuropathic pain treated with tramadol. Rev Bras Anestesiol. 2009; 59:297–303. PMID: 19488542.

11. Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 2013; 3:175–184. PMID: 24436868.

12. Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A, Norval M. Local immune responses and systemic cytokine responses in zoster: relationship to the development of postherpetic neuralgia. Clin Exp Immunol. 2003; 131:318–323. PMID: 12562395.

13. Bäckryd E, Ghafouri B, Larsson B, Gerdle B. Plasma pro-inflammatory markers in chronic neuropathic pain: A multivariate, comparative, cross-sectional pilot study. Scand J Pain. 2016; 10:1–5. PMID: 28361755.

14. Zhu SM, Liu YM, An ED, Chen QL. Influence of systemic immune and cytokine responses during the acute phase of zoster on the development of postherpetic neuralgia. J Zhejiang Univ Sci B. 2009; 10:625–630. PMID: 19650202.

15. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006; 8(Suppl 2):S3.
16. Karadag Oncel E, Kara A, Celik M, Karahan S, Cengiz AB, Ceyhan M. Determination and clinical correlation of markers of inflammation in unvaccinated patients with varicellazoster infection. Eur Rev Med Pharmacol Sci. 2013; 17:2032–2039. PMID: 23884823.
17. Desmond RA, Weiss HL, Arani RB, Soong SJ, Wood MJ, Fiddian PA, et al. Clinical applications for change-point analysis of herpes zoster pain. J Pain Symptom Manage. 2002; 23:510–516. PMID: 12067775.

Fig. 1
Receiver operating characteristic curves of the models adding each inflammatory marker (red) compared with that of a reference model using age and initial pain visual analogue scale (blue, area under curve [AUC]=0.85); (A) erythrocyte sedimentation rate (AUC=0.90, p=0.049), (B) C-reactive protein (AUC=0.89, p=0.221), and (C) lymphocyte count (AUC=0.88, p=0.284).
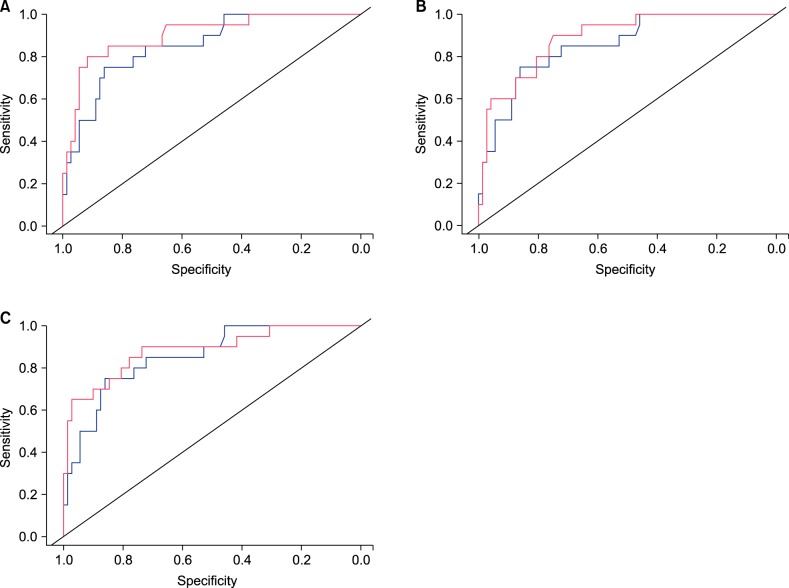
Table 1
Demographic, clinical, and laboratory characteristics of study participants by follow-up visit
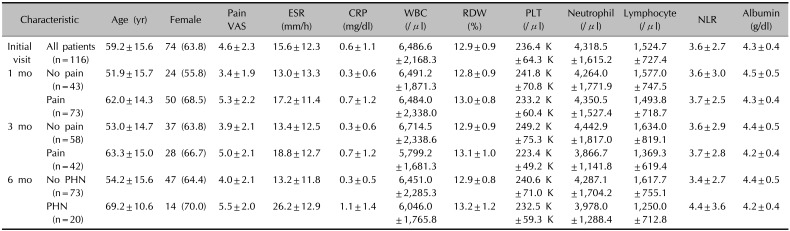
Table 2
Univariate and multivariate analysis of each inflammatory marker and the presence or absence of postherpetic neuralgia at 6 months after diagnosis of herpes zoster
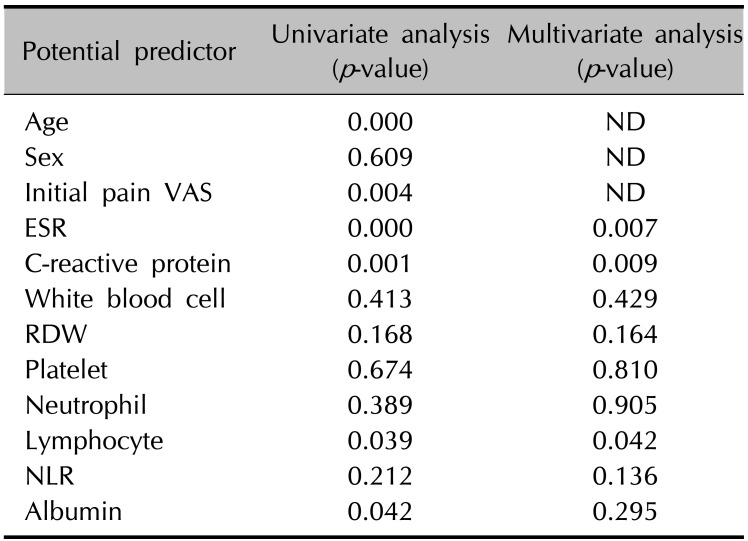
Table 3
Multivariate analyses in each model including each statistically significant inflammatory marker for development of postherpetic neuralgia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download