INTRODUCTION
MATERIALS AND METHODS
Subjects
Methods
Measurement of cytokine levels
Assessments of therapeutic effects and adverse events
Assessment of changes in cytokine levels
Statistical analysis
RESULTS
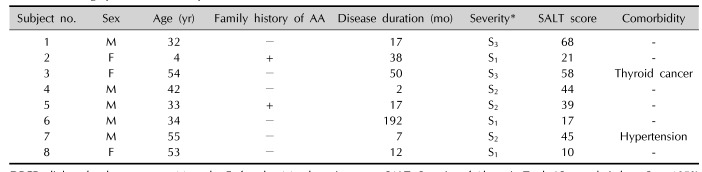
Demographic data
Table 1
Demographic data of the patients treated with DPCP
Objective assessment
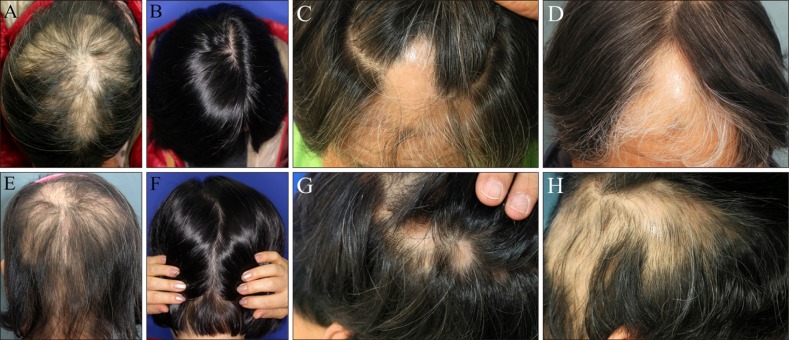 | Fig. 1Gross photograph of patient no. 3 (responder) and patient no. 7 (non-responder) at before and 4 months after DPCP treatment. Before treatment (A, E: patient no. 3; C, G: patient no. 7). After 4 months of treatment (B, F: patient no. 3; D, H: patient no. 7). |
Table 2
Summary of clinical results of the patients treated with DPCP
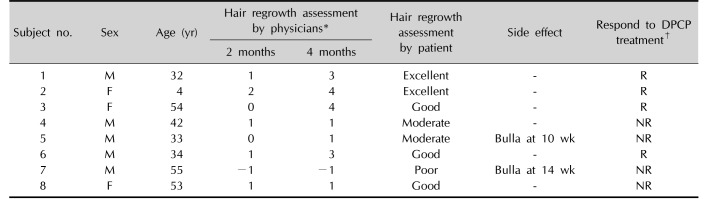
DPCP: diphenylcyclopropenone, M: male, F: female, R: responder, NR: non-responder. *−1=spreading, 0=no hair growth, 1=1%~24% hair growth, 2=25%~49% hair growth, 3=50%~74% hair growth, 4=75%~99% hair growth, 5=100% hair growth. †Responder: hair regrowth assessment score ≥3 (hair regrowth ≥50%) at 4 months after DPCP treatment, non-responder: hair regrowth assessment score <3 (hair regrowth <50%) at 4 months after DPCP treatment.
Subjective satisfaction assessment
Adverse events assessments
Changes in cytokine levels
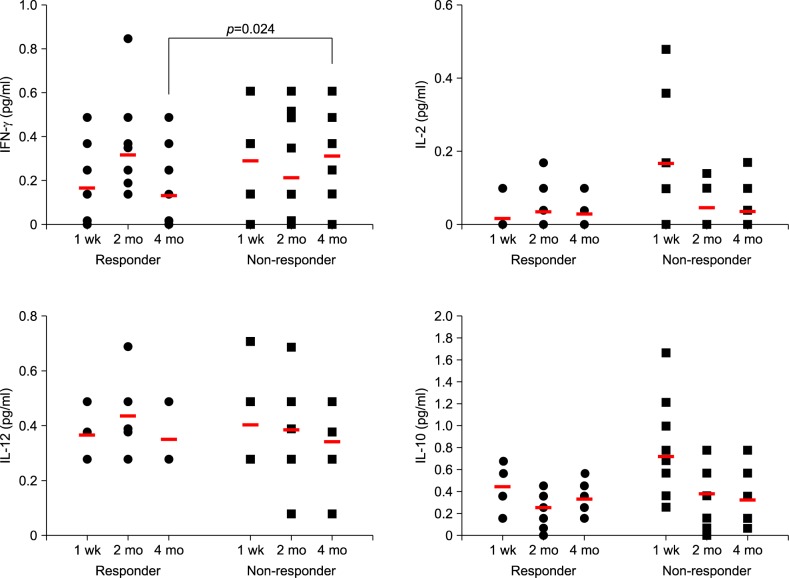 | Fig. 2Dot plot showing each value of cytokines in the patients treated with diphenylcyclopropenone (DPCP). Red bar in the graph indicates mean values. Before significant clinical differences appear (1 week and 2 months after treatment), interferon (IFN)-gamma, interleukin (IL)-12, and IL-10 showed no significant difference between responders and non-responders. IL-2 levels at 1 week showed difference between the two groups, however, it did not showed difference at 4 months after treatment. IFN-gamma levels at 4 months after DPCP treatment showed statistically significant differences between responder and non-responder groups (p=0.024). |
Table 3
Comparison of the cytokine levels between responder and non-responder groups at 1 week, 2 months, and 4 months
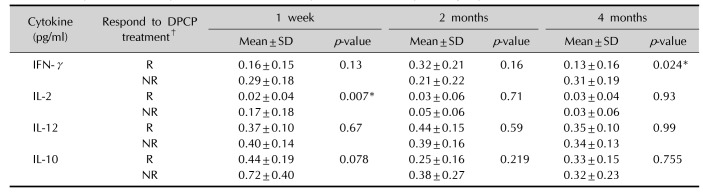
Values are presented as mean±standard deviation (SD) for n=4 of the three independent experiments. DPCP: diphenylcyclopropenone, IFN: interferon, IL: interleukin, R: responder (n=4), NR: non-responder (n=4). *p<0.05 calculated by Mann-Whitney U-test between responder and non-responder groups. †Responder: hair regrowth assessment score ≥3 (hair regrowth ≥50%) at 4 months after DPCP treatment, non-responder: hair regrowth assessment score <3 (hair regrowth <50%) at 4 months after DPCP treatment.
Table 4
Comparison of the differences of cytokine levels in each group after DPCP treatment
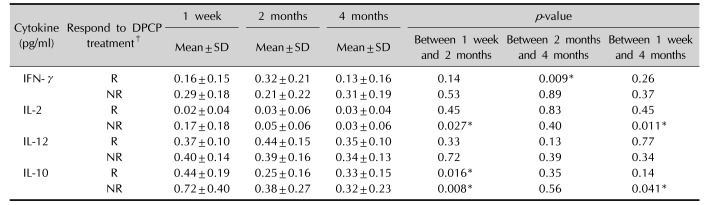
Values are presented as mean±standard deviation (SD) of changes between each time intervals, for n=4 of the three independent experiments. DPCP: diphenylcyclopropenone, IFN: interferon, IL: interleukin, R: responder (n=4), NR: non-responder (n=4). *p<0.05 calculated by Wilcoxon signal rank test in the responder and non-responder group each. †Responder: hair regrowth assessment score ≥3 (hair regrowth ≥50%) at 4 months after DPCP treatment, non-responder: hair regrowth assessment score <3 (hair regrowth <50%) at 4 months after DPCP treatment.
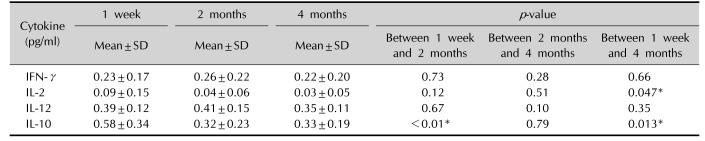
Table 5
Comparison of the differences of cytokine levels in total eight patients after DPCP treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download