Abstract
Background and Purpose
Epilepsy is a chronic neurological disease that represents a tremendous burden on both patients and society in general. Studies have addressed how demographic variables, socioeconomic variables, and psychological comorbidity are related to the quality of life (QOL) of people with epilepsy (PWE). However, there has been less focus on how these factors may differ between patients who exhibit varying degrees of seizure control. This study utilized data from the Managing Epilepsy Well (MEW) Network of the Centers for Disease Control and Prevention with the aim of elucidating differences in demographic variables, depression, and QOL between adult PWE.
Methods
Demographic variables, depression, and QOL were compared between PWE who experience clinically relevant differences in seizure occurrence.
Results
Gender, ethnicity, race, education, income, and relationship status did not differ significantly between the seizure-frequency categories (p>0.05). People with worse seizure control were significantly younger (p=0.039), more depressed (as assessed using the Patient Health Questionnaire) (p=0.036), and had lower QOL (as determined using the 10-item Quality of Life in Epilepsy for Adults scale) (p<0.001).
Epilepsy is a chronic debilitating condition that affects about 2.9 million people in the United States.1 The disorder represents a tremendous burden on both individuals and society, with estimates of a direct cost of USD 4.3 billion and a total cost of USD 9.6–15.5 billion annually.23 Although the disease is characterized by episodic attacks, unpredictable recurrent seizures in people with epilepsy (PWE) cause significant disability and negatively impact their health, productivity, and activities of daily living.45 Further, the disease has substantial psychosocial, behavioral, and cognitive sequelae, with frequent seizures, stigma, and seizure worry associated with reduced quality of life (QOL).678 Requirements for chronic medication, barriers to care, employment limitations, and inability to drive a motor vehicle represent longterm hindrances for PWE.
Numerous studies have addressed the impact of demographic and socioeconomic variables, seizure frequency, and comorbid depression on health-related QOL in PWE,91011121314 but there has been less research into how these factors may differ among PWE who have varying clinically relevant degrees of seizure control (i.e., completely controlled, occasional seizures, or poorly controlled). Reports on factors affecting seizure frequency in PWE have typically focused on pharmacological treatments15 or outcomes of epilepsy surgery.16 Since PWE are a heterogeneous population in which the frequency of seizures may vary significantly between individuals and seizure control is a major therapeutic objective, there is value in identifying differences in demographic and clinical variables between PWE with varying seizure frequencies.
The primary purpose of this study was to elucidate differences in demographic variables, depression, and QOL between PWE stratified by seizure frequency in a non-surgical sample of adult PWE using aggregate, de-identified data obtained in studies conducted at multiple United States institutions coordinated by the Managing Epilepsy Well (MEW) Network of the Centers for Disease Control and Prevention (https://www.cdc.gov/epilepsy/research/MEW-network.htm and http://www.managingepilepsywell.org). The present study additionally aimed to demonstrate the validity of using the MEW Network Database (MEW-DB) to conduct research on epilepsy. The MEW-DB is a harmonized database that includes de-identified data obtained in studies of self-management in PWE. The MEW-DB was used to compare the variables of interest between PWE in the following three clinically relevant groups categorized based on the seizure frequency during the past 30 days: completely seizure-free (SZF); fair seizure control, with one to four seizures (FC); and poor seizure control, with at least five seizures (PC).
This cross-sectional analysis used data from 481 PWE enrolled in 6 MEW Network epilepsy self-management studies. A detailed description of the MEW Network is available elsewhere.17 The MEW-DB was developed to facilitate aggregate secondary analyses of data collected at individual MEW Network sites,18 with approval received from the Institutional Review Board of University Hospitals Cleveland Medical Center (Principal Investigator, Martha Sajatovic; Reference no. 068620). The de-identified data reported here were collected across four U.S. institutions using studies performed from 2007 to 2016 and reports on baseline data only. The aggregate data used in the analysis comprised participant data from the HOBSCOTCH (HOme-Based Self-management and Cognitive Training CHanges lives) clinical trial performed at Dartmouth-Hitchcock Medical Center,19 the WebEase (Web Epilepsy Awareness Support and Education) clinical trial performed at Emory University,20 the FOCUS (Figure out the problem or the issue, Observe your routine, Connect your observations and choose a change goal, Undertake a change strategy, and Study the results) on Epilepsy pilot study and randomized controlled trial performed at the University of Michigan, and the TIME (Targeted Self-Management for Epilepsy and serious mental illness) and SMART (Self-management for people with epilepsy and a history of negative health events) clinical trials performed at Case Western Reserve University. All of the included individuals were at least 18 years old and had a self-reported diagnosis of epilepsy. They provided informed consent for participating in the respective studies and the values of demographic variables including age, gender, race, ethnicity, education, income, and relationship status. The study participants also provided information on seizure frequency and were assessed for QOL and depression severity. Seizure frequency was standardized across studies to a consistent time frame of 30 days prior to presentation for the baseline study assessment. Data on seizure type and epilepsy type were not consistently available across the included studies. The patients were classified based on the 30-day seizure count into SZF, FC, and PC groups.
QOL in this study was measured with the 10-item Quality of Life in Epilepsy for Adults scale (QOLIE-10). The QOLIE-10 is an extensively validated survey of health-related QOL that covers both general and epilepsy-specific domains.21 It is used by clinicians and researchers in various settings and languages, and is a screening version derived from 2 longer instruments: the 89-item QOLIE-89 and the 31-item QOLIE-31. The QOLIE-10 assessment includes questions on health, daily activities, and distress related to epilepsy. Total scores on the QOLIE-10 range from 1 to 50, with lower scores indicating better QOL. To accommodate missing data in the pooled MEW-DB sample, the total score for QOLIE-10 was calculated as the sum of scores for all questions divided by the number of questions answered and a score range of 1–5 with lower scores indicating better QOL. Previous studies have investigated the correlations between QOLIE-10 scores and health-care utilization,22 satisfaction with care,23 anxiety,8 and depression.8
Depression in this study was quantified with the nine-item Patient Health Questionnaire (PHQ-9), which is a self-administered multipurpose instrument for screening, measuring, and monitoring the severity of depression in patients. The PHQ-9 has been validated in several patient populations,24 including adult PWE,25 and it incorporates the Diagnostic and Statistical Manual of Mental Disorders-5 diagnostic criteria for major depressive disorder in patients with medical comorbidities. In PWE, the PHQ-9 has been validated against the Neurological Disorders Depression Inventory for Epilepsy25 and has been used in several studies.2627 Scores on the PHQ-9 of 5, 10, 15, and 20 correspond to mild, moderate, moderately severe, and severe depression, respectively.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) program (version 22, IBM SPSS Statistics for Windows, IBM Corporation, Armonk, NY, USA). Due to heterogeneity in variable definitions and data collection between the 4 study sites contributing data, the sample sizes used when comparing the demographic variables and health status measures between the 3 clinically relevant seizure-frequency groups (SZF, FC, and PC) differed between variables, ranging from 481 for age to 230 for relationship status (Table 1). Chi-square tests were used to compare the categorical variables of gender, race, ethnicity, education, income, and relationship status. One-way ANOVA was applied to identify significant intergroup differences in the continuous variables of age, PHQ-9 score, and QOLIE-10 score. A post-hoc analysis of differences between groups was conducted using Tukey's honest-significant-difference test. In addition to evaluating the three clinically relevant seizure-frequency subgroups, demographic variables and clinical correlates of the 30-day seizure frequency as a continuous measure were evaluated using Spearman's or Pearson's correlation for continuous clinical measures (i.e., age, PHQ-9, QOLIE-10), and t-tests, Kruskal-Wallis tests, or Mann-Whitney tests for categorical clinical measures. The cutoff for statistical significance in this study was set at p≤0.05.
The demographic and clinical variables of the entire sample and the three seizure-frequency categories are summarized and compared in Table 1. The participants were aged 42.8±13.0 years (mean±standard deviation, n=481). Of the 480 participants who reported gender, 157 (32.7%) of them were male and 323 (67.3%) were female. Of the 272 participants who reported ethnicity, 25 (9.2%) identified as Hispanic. Of the 405 participants who reported their race, 72 (17.8%) were African-American. The sample of PWE for whom data on education were available comprised 476 individuals, 369 (77.5%) of whom were college educated. As for income, out of 232 included PWE, 148 (63.8%) reported annual earnings of less than USD 25,000, 33 (14.2%) earned USD 25,000–49,999, and 51 (22.0%) had incomes of at least USD 50,000. In terms of marital or relationship status, 93 out of 230 (40.4%) PWE reported being married or partnered. The PHQ-9 score in 331 PWE was 8.9±6.2, and the QOLIE-10 score in 415 PWE was 2.9±0.9.
Gender, ethnicity, race, education, income, and relationship status did not differ significantly between PWE in the SZF, FC, and PC groups. Patients with worse seizure control were younger (p<0.039), significantly more depressed (p=0.036), and had a lower QOL (p<0.001). Those in the SZF, FC, and PC groups were aged 44.6±13.7, 42.7±12.8, and 40.0±11.8 years, respectively (p=0.039); their PHQ-9 scores were 7.6±5.4, 9.4±6.4, and 9.8±7.0 (p=0.036), and their QOLIE-10 scores were 2.5±0.8, 3.1±0.9, and 3.2±0.7 (p<0.001) (Fig. 1).
Our evaluation of the relationship between the past 30-day seizure frequency as a continuous measure (irrespective of the clinically relevant degree of seizure control) revealed that PHQ-9 and QOLIE-10 scores remained significantly correlated (rho=0.19 and p=0.001, and rho=0.381 and p<0.001, respectively). Greater number of seizures was associated with worse depression severity and lower QOL. Age was not significantly correlated with 30-day seizure frequency (rho=−0.02, p=0.67). With respect to categorical demographic variables, we found that 30-day seizure frequency as a continuous variable was significantly correlated with gender (p=0.047) but not with the other demographic variables.
This study found that PWE with different clinically relevant degrees of seizure control generally have similar demographic characteristics. However, evidence was found of significant differences in depression and QOL in PWE, with frequent seizures associated with an increased severity of depression and lower QOL. Notably, the PHQ-9 and QOLIE-10 scores did not differ significantly between the FC and PC groups, but they were significantly different in the SZF group, suggesting that experiencing a seizure over a 30-day period is associated with worse QOL in PWE. This underscores the importance of achieving complete seizure freedom for ameliorating depression and improving the QOL in this patient population.
The cross-sectional design of this study means that causal inferences cannot be made, but the elucidated relationship between seizure frequency and QOL is congruous with the results of numerous published multivariate analyses of QOL correlates in PWE. Other studies have shown that both depression1112 and seizure frequency10121314 are correlated with reduced QOL in PWE. Poor seizure control might precipitate stress and depression, leading to reduced QOL. The consistency of the present findings with those in other populations of PWE underscores the validity of using the MEW-DB to advance original clinical research into epilepsy.
The significant inverse relationship found between age and seizure frequency in the three frequency categories of PWE might reflect how chronic illness is managed. Learning to manage epilepsy takes time and practice, as supported by a recent prospective study of seizure recurrence in PWE treated with antiepileptic monotherapy finding older age to be associated with a lower likelihood of seizure at follow-up.28 Additionally, Escoffery et al.29 found that safety, wellness, and treatment behaviors were performed less by younger PWE (<30 years) compared to those in older age groups. However, our analysis of the 30-day seizure frequency as a continuous measure in relation to demographic variables did not show a significant relationship with age. These discrepancies might have been due to our sample being relatively small, with limited numbers of individuals at the upper and lower extremes of age.
The lack of significant differences in other demographic variables such as education and income between the SZF group and the FC and PC groups suggests that these factors may be more affected by the affective and cognitive sequelae of epilepsy than by the frequency of seizures.30 Since the mortality, morbidity, and long-term prognosis in chronic disease are negatively impacted by the presence of affective disorders, depression can be conceptualized as a modifiable factor in seizure frequency and QOL outcomes in epilepsy. Comorbid depression has been shown to cause poor medication adherence,31 which in PWE can increase the frequency and severity seizures and decrease QOL. A significant association has been shown between seizure frequency and QOL across all subscale domains of the QOLIE-31 assessment: seizure worry, overall QOL, emotional well-being, energy/fatigue, cognitive functioning, medication effect, and social functioning.9 The rate of psychiatric illness is higher in PWE than in the general population and those affected by other forms of chronic illness.3233
Our findings underscore the importance of the timely identification of comorbid depression by applying screening tools such as the PHQ-9, with subsequent behavioral therapy and/or pharmacological management being applied where required. The benefit of early depression screening, detection, and treatment in PWE has been demonstrated by other investigators. In a randomized controlled trial of systemic family therapy in younger PWE and comorbid depression and anxiety, Li et al.34 found that therapy significantly reduced the 30-day seizure frequency as well as the depression and anxiety symptoms.
Several MEW Network interventions specifically target the reduction of depression as a modifiable and important outcome in epilepsy self-management.35 The UPLIFT (Using Practice and Learning to Increase Favorable Thoughts) for Prevention program tested a remotely delivered self-management intervention for PWE with mild symptoms of depression. The trial demonstrated that the incidence of new or relapsing depressive episodes was significantly lower in the intervention group than in the controls.36 These results supplemented those from a previous single-site trial of UPLIFT for Treatment.37 In another MEW Network study, Ciechanowski et al.38 implemented the Program to Encourage Active, Rewarding Lives for Seniors (PEARLS), an evidence-based treatment program for depression in older adults and PWE.39 Adults participating in the PEARLS program exhibited significantly lower severities of depression and suicidal ideation.3839 The recently published TIME study targeting PWE with comorbid severe mental illnesses including severe depression, schizophrenia, and bipolar disorder found that TIME was associated with improved depression severity compared to the standard treatment.40
Limitations of the present study include its cross-sectional design and the self-reporting of data from heterogeneous sources, which led to inconsistencies in the sample sizes and in the various demographic and clinical variables analyzed between the included studies. Additionally, the education level of the included participants was on average higher than the typical level in large studies of PWE. However, at least half of the study participants were from lower income households. The higher level of education might reflect selection bias in PWE who enroll in self-management research. The high prevalence of moderate depression also reflects selection bias for study participation.
The continuing expansion and refinement of the MEW-DB will led to future analyses aimed at characterizing the longitudinal outcomes for variations in seizure frequency, mood, and other health outcomes among individuals with a chronic neurological condition.
Acknowledgements
This study was funded by the Centers for Disease Control and Prevention (CDC) (grant no. 3U48DP001935-04S3) and the Managing Epilepsy Well Network of the CDC. The MEW Network is funded by the CDC and supported by special interest projects SIP 14-006 and SIP 14-007. The Cooperative Agreement Numbers are 1U48DP005002 (University of Arizona), 1U48DP005018 (Geisel School of Medicine at Dartmouth), 1U48DP005010 (University of Illinois), 1U48DP005042 (Morehouse), U48DP005008 (New York University), 1U48DP005022 (University of Minnesota), 1U48DP005030 (Case Western Reserve), and 148DP005013 (University of Washington).
References
1. Kobau R, Luo Y, Zack MM, Helmers S, Thurman DJ. Centers for Disease Control and Prevention (CDC). Epilepsy in adults and access to care–United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 61:909–913. PMID: 23151949.
2. Yoon D, Frick KD, Carr DA, Austin JK. Economic impact of epilepsy in the United States. Epilepsia. 2009; 50:2186–2191. PMID: 19508694.

3. Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016; 316:2627–2646. PMID: 28027366.

4. Kleen JK, Scott RC, Lenck-Santini PP, Holmes GL. Cognitive and behavioral comorbidities of epilepsy. In : Noebels JL, Avoli M, Rogawski MA, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Bethesda: National Center for Biotechnology Information (US);2012. p. 915–929.
5. Lehrner J, Kalchmayr R, Serles W, Olbrich A, Pataraia E, Aull S, et al. Health-related quality of life (HRQOL), activity of daily living (ADL) and depressive mood disorder in temporal lobe epilepsy patients. Seizure. 1999; 8:88–92. PMID: 10222299.

6. Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav. 2004; 5:976–980. PMID: 15582847.

7. Baker GA, Brooks J, Buck D, Jacoby A. The stigma of epilepsy: a European perspective. Epilepsia. 2000; 41:98–104. PMID: 10643931.

8. Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004; 45:544–550. PMID: 15101836.

9. Djibuti M, Shakarishvili R. Influence of clinical, demographic, and socioeconomic variables on quality of life in patients with epilepsy: findings from Georgian study. J Neurol Neurosurg Psychiatry. 2003; 74:570–573. PMID: 12700294.

10. Vickrey BG, Berg AT, Sperling MR, Shinnar S, Langfitt JT, Bazil CW, et al. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia. 2000; 41:760–764. PMID: 10840410.

11. Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003; 4(Suppl 4):S26–S30. PMID: 14654425.

12. Azuma H, Akechi T. Effects of psychosocial functioning, depression, seizure frequency, and employment on quality of life in patients with epilepsy. Epilepsy Behav. 2014; 41:18–20. PMID: 25269689.

13. van Hout B, Gagnon D, Souêtre E, Ried S, Remy C, Baker G, et al. Relationship between seizure frequency and costs and quality of life of outpatients with partial epilepsy in France, Germany, and the United Kingdom. Epilepsia. 1997; 38:1221–1226. PMID: 9579924.

14. McLachlan RS, Rose KJ, Derry PA, Bonnar C, Blume WT, Girvin JP. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997; 41:482–489. PMID: 9124805.

15. Lortie A, Chiron C, Mumford J, Dulac O. The potential for increasing seizure frequency, relapse, and appearance of new seizure types with vigabatrin. Neurology. 1993; 43(11 Suppl 5):S24–S27. PMID: 8232984.
16. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001; 345:311–318. PMID: 11484687.

17. Sajatovic M, Jobst BC, Shegog R, Bamps YA, Begley CE, Fraser RT, et al. The managing epilepsy well network: advancing epilepsy self-management. Am J Prev Med. 2017; 52(3S3):S241–S245. PMID: 28215372.
18. Sahoo SS, Zhang GQ, Bamps Y, Fraser R, Stoll S, Lhatoo SD, et al. Managing information well: toward an ontology-driven informatics platform for data sharing and secondary use in epilepsy self-management research centers. Health Informatics J. 2016; 22:548–561. PMID: 25769938.

19. Caller TA, Ferguson RJ, Roth RM, Secore KL, Alexandre FP, Zhao W, et al. A cognitive behavioral intervention (HOBSCOTCH) improves quality of life and attention in epilepsy. Epilepsy Behav. 2016; 57:111–117. PMID: 26943948.

20. DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav. 2011; 22:469–474. PMID: 21889413.

21. Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia. 1996; 37:577–582. PMID: 8641236.

22. Bautista RE, Glen ET, Wludyka PS, Shetty NK. Factors associated with utilization of healthcare resources among epilepsy patients. Epilepsy Res. 2008; 79:120–129. PMID: 18339521.

23. Bautista RE, Glen ET, Shetty NK. Factors associated with satisfaction with care among patients with epilepsy. Epilepsy Behav. 2007; 11:518–524. PMID: 17936688.

24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606–613. PMID: 11556941.
25. Rathore JS, Jehi LE, Fan Y, Patel SI, Foldvary-Schaefer N, Ramirez MJ, et al. Validation of the Patient Health Questionnaire-9 (PHQ-9) for depression screening in adults with epilepsy. Epilepsy Behav. 2014; 37:215–220. PMID: 25064739.

26. Seminario NA, Farias ST, Jorgensen J, Bourgeois JA, Seyal M. Determination of prevalence of depression in an epilepsy clinic using a brief DSM-IV-based self-report questionnaire. Epilepsy Behav. 2009; 15:362–366. PMID: 19525150.

27. Fiest KM, Patten SB, Altura KC, Bulloch AG, Maxwell CJ, Wiebe S, et al. Patterns and frequency of the treatment of depression in persons with epilepsy. Epilepsy Behav. 2014; 39:59–64. PMID: 25203325.

28. Shcherbakova N, Rascati K, Brown C, Lawson K, Novak S, Richards KM, et al. Factors associated with seizure recurrence in epilepsy patients treated with antiepileptic monotherapy: a retrospective observational cohort study using US administrative insurance claims. CNS Drugs. 2014; 28:1047–1058. PMID: 25086640.

29. Escoffery C, McGee RE, Bamps Y, Helmers SL. Differences in epilepsy self-management behaviors among young and older adults. Austin J Neurol Disord Epilepsy. 2016; 3:1015.
30. Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999; 67:44–50. PMID: 10369821.

31. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007; 3:117–131. PMID: 19300542.

32. Kerr MP, Mensah S, Besag F, de Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011; 52:2133–2138. PMID: 21955156.

33. LaFrance WC Jr, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. Int Rev Neurobiol. 2008; 83:347–383. PMID: 18929092.
34. Li J, Wang X, Meng H, Zeng K, Quan F, Liu F. Systemic family therapy of comorbidity of anxiety and depression with epilepsy in adolescents. Psychiatry EInvestig. 2016; 13:305–310.

35. DiIorio CK, Bamps YA, Edwards AL, Escoffery C, Thompson NJ, Begley CE, et al. The prevention research centers’ managing epilepsy well network. Epilepsy Behav. 2010; 19:218–224. PMID: 20869323.

36. Thompson NJ, Patel AH, Selwa LM, Stoll SC, Begley CE, Johnson EK, et al. Expanding the efficacy of Project UPLIFT: distance delivery of mindfulness-based depression prevention to people with epilepsy. J Consult Clin Psychol. 2015; 83:304–313. PMID: 25495361.

37. Thompson NJ, Walker ER, Obolensky N, Winning A, Barmon C, Diiorio C, et al. Distance delivery of mindfulness-based cognitive therapy for depression: project UPLIFT. Epilepsy Behav. 2010; 19:247–254. PMID: 20851055.

38. Ciechanowski P, Chaytor N, Miller J, Fraser R, Russo J, Unutzer J, et al. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav. 2010; 19:225–231. PMID: 20609631.

39. Chaytor N, Ciechanowski P, Miller JW, Fraser R, Russo J, Unutzer J, et al. Long-term outcomes from the PEARLS randomized trial for the treatment of depression in patients with epilepsy. Epilepsy Behav. 2011; 20:545–549. PMID: 21333607.

40. Sajatovic M, Tatsuoka C, Welter E, Perzynski AT, Colon-Zimmermann K, Van Doren JR, et al. Targeted self-management of epilepsy and mental illness for individuals with epilepsy and psychiatric comorbidity. Epilepsy Behav. 2016; 64:152–159. PMID: 27743547.

Fig. 1
Age and QOLIE-10 and PHQ-9 scores among adult patients with epilepsy enrolled in Managing Epilepsy Well Network studies. A: Age. B: Total PHQ-9 score. C: QOLIE-10 score. Data are mean and standard-deviation values. *p value significant at p≤0.05, †p value significant at p≤0.01. FC: one to four recent seizures, PC: at least five recent seizures, PHQ-9: nine-item Patient Health Questionnaire (lower scores indicate less severe depression symptoms), QOLIE-10: 10-item Quality of Life in Epilepsy for Adults scale (lower scores indicate better quality of life), SZF: no recent seizures.
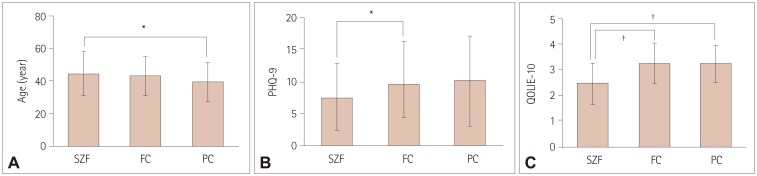
Table 1
Comparison of baseline demographic and clinical variables stratified by 30-day seizure frequency among adult patients with epilepsy in the Managing Epilepsy Well Network
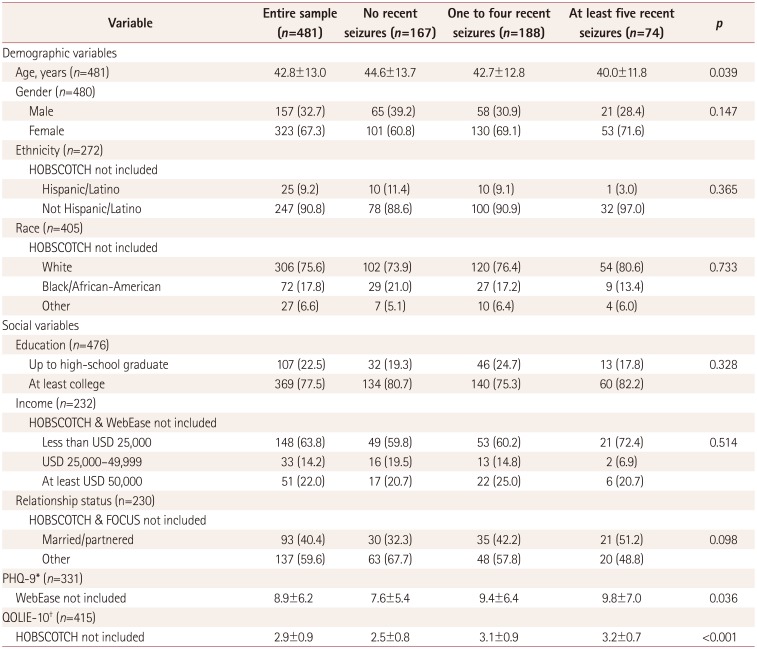
Data are n (%) or mean±standard-deviation values.
*Lower scores indicate less-severe depression, †Lower scores indicate better quality of life.
FOCUS: Figure out the problem or the issue, Observe your routine, Connect your observations and choose a change goal, Undertake a change strategy, and Study the results, HOBSCOTCH: HOme-Based Self-management and Cognitive Training CHanges lives, PHQ-9: nine-item Patient Health Questionnaire, QOLIE-10: 10-item Quality of Life in Epilepsy for Adults scale, WebEase: Web Epilepsy Awareness Support and Education.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download