1. The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993; 43:655–661. PMID:
8469318.
2. Deisenhammer F. Neutralizing antibodies to interferon-beta and other immunological treatments for multiple sclerosis: prevalence and impact on outcomes. CNS Drugs. 2009; 23:379–396. PMID:
19453200.
3. Sorensen PS, Koch-Henriksen N, Ross C, Clemmesen KM, Bendtzen K. Danish Multiple Sclerosis Study Group. Appearance and disappearance of neutralizing antibodies during interferon-beta therapy. Neurology. 2005; 65:33–39. PMID:
15888603.

4. Rudick RA, Simonian NA, Alam JA, Campion M, Scaramucci JO, Jones W, et al. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology. 1998; 50:1266–1272. PMID:
9595973.
5. Francis GS, Rice GP, Alsop JC. PRISMS Study Group. Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology. 2005; 65:48–55. PMID:
16009884.
6. Sorensen PS, Ross C, Clemmesen KM, Bendtzen K, Frederiksen JL, Jensen K, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 2003; 362:1184–1191. PMID:
14568740.

7. Hegen H, Millonig A, Bertolotto A, Comabella M, Giovanonni G, Guger M, et al. Early detection of neutralizing antibodies to interferon-beta in multiple sclerosis patients: binding antibodies predict neutralizing antibody development. Mult Scler. 2014; 20:577–587. PMID:
24009164.

8. Cepok S, Schreiber H, Hoffmann S, Zhou D, Neuhaus O, von Geldern G, et al. Enhancement of chemokine expression by interferon beta therapy in patients with multiple sclerosis. Arch Neurol. 2009; 66:1216–1223. PMID:
19667211.

9. Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014; 89:225–240. PMID:
24485135.

10. Río J, Comabella M, Montalban X. Predicting responders to therapies for multiple sclerosis. Nat Rev Neurol. 2009; 5:553–560. PMID:
19794514.

11. Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol. 2009; 8:545–559. PMID:
19446274.

12. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302. PMID:
21387374.

13. Hyun JW, Kim SH, Jeong IH, Ahn SW, Huh SY, Park MS, et al. Utility of the rio score and modified rio score in Korean patients with multiple sclerosis. PLoS One. 2015; 10:e0129243. PMID:
26010897.

14. Creeke PI, Farrell RA. Clinical testing for neutralizing antibodies to interferon-β in multiple sclerosis. Ther Adv Neurol Disord. 2013; 6:3–17. PMID:
23277789.

15. Govindappa K, Sathish J, Park K, Kirkham J, Pirmohamed M. Development of interferon beta-neutralising antibodies in multiple sclerosis--a systematic review and meta-analysis. Eur J Clin Pharmacol. 2015; 71:1287–1298. PMID:
26268445.

16. Bertolotto A, Malucchi S, Milano E, Castello A, Capobianco M, Mutani R. Interferon beta neutralizing antibodies in multiple sclerosis: neutralizing activity and cross-reactivity with three different preparations. Immunopharmacology. 2000; 48:95–100. PMID:
10936507.
17. Pachner A, Narayan K, Price N, Hurd M, Dail D. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn. 2003; 7:17–25. PMID:
14529316.
18. Bertolotto A, Sala A, Caldano M, Capobianco M, Malucchi S, Marnetto F, et al. Development and validation of a real time PCR-based bioassay for quantification of neutralizing antibodies against human interferon-beta. J Immunol Methods. 2007; 321:19–31. PMID:
17335844.

19. Farrell R, Kapoor R, Leary S, Rudge P, Thompson A, Miller D, et al. Neutralizing anti-interferon beta antibodies are associated with reduced side effects and delayed impact on efficacy of interferon-beta. Mult Scler. 2008; 14:212–218. PMID:
17986510.

20. Lam R, Farrell R, Aziz T, Gibbs E, Giovannoni G, Grossberg S, et al. Validating parameters of a luciferase reporter gene assay to measure neutralizing antibodies to IFNbeta in multiple sclerosis patients. J Immunol Methods. 2008; 336:113–118. PMID:
18511063.
21. Sørensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, et al. Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFNbeta antibodies in multiple sclerosis. Eur J Neurol. 2005; 12:817–827. PMID:
16241970.

22. Polman CH, Bertolotto A, Deisenhammer F, Giovannoni G, Hartung HP, Hemmer B, et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 2010; 9:740–750. PMID:
20610349.

23. Bertolotto A, Capobianco M, Amato MP, Capello E, Capra R, Centonze D, et al. Guidelines on the clinical use for the detection of neutralizing antibodies (NAbs) to IFN beta in multiple sclerosis therapy: report from the Italian Multiple Sclerosis Study group. Neurol Sci. 2014; 35:307–316. PMID:
24374787.

24. Hesse D, Sørensen PS. Using measurements of neutralizing antibodies: the challenge of IFN-beta therapy. Eur J Neurol. 2007; 14:850–859. PMID:
17662004.
25. Goodin DS, Frohman EM, Hurwitz B, O'Connor PW, Oger JJ, Reder AT, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007; 68:977–984. PMID:
17389300.

26. Buttmann M, Merzyn C, Rieckmann P. Interferon-beta induces transient systemic IP-10/CXCL10 chemokine release in patients with multiple sclerosis. J Neuroimmunol. 2004; 156:195–203. PMID:
15465611.
27. Lünemann JD, Waiczies S, Ehrlich S, Wendling U, Seeger B, Kamradt T, et al. Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto) antigen-specific T cells. J Immunol. 2002; 168:4881–4888. PMID:
11994437.
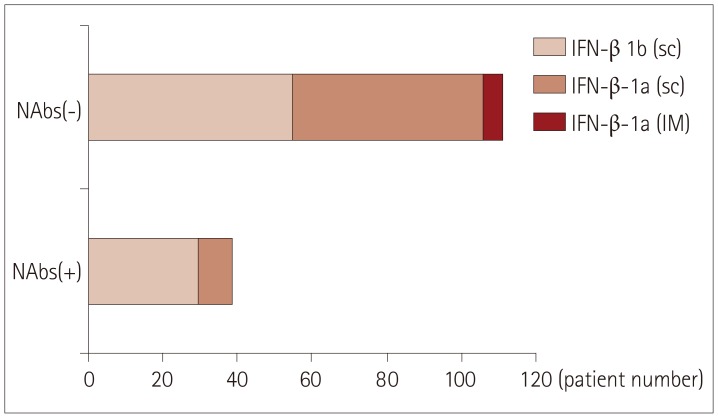
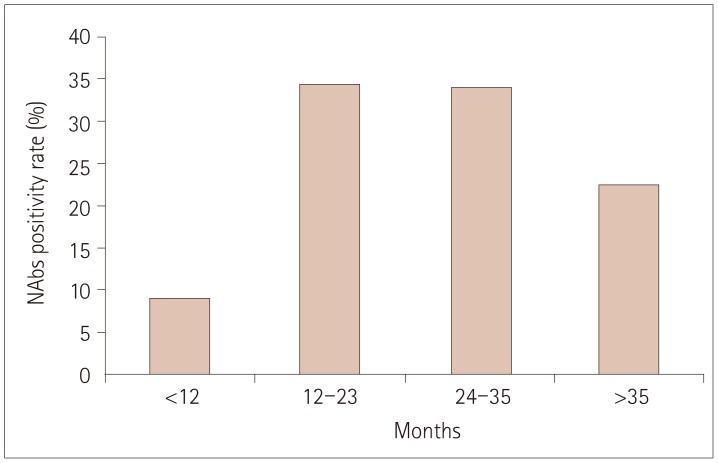

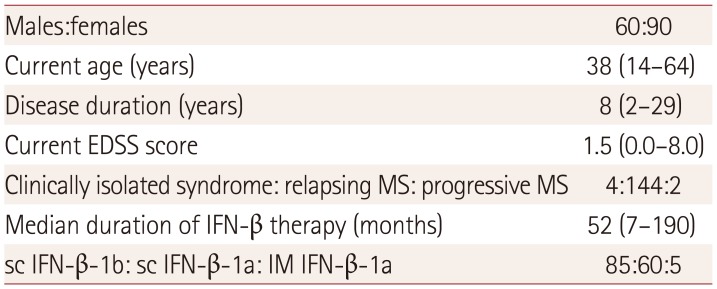
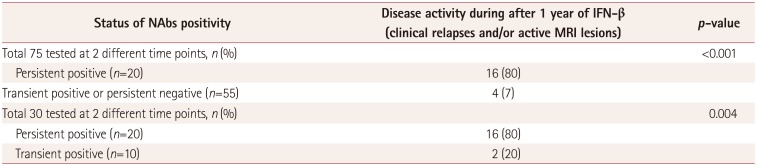




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download