Abstract
Background and Purpose
The literature indicates that obstructive sleep apnea (OSA) increases the risk of ischemic stroke. However, the causal relationship between OSA and ischemic stroke is not well established. This study examined whether preexisting OSA symptoms affect the onset of acute ischemic stroke.
Methods
We investigated consecutive patients who were admitted with acute ischemic stroke, using a standardized protocol including the Berlin Questionnaire on symptoms of OSA prior to stroke. The collected stroke data included the time of the stroke onset, risk factors, and etiologic subtypes. The association between preceding OSA symptoms and wake-up stroke (WUS) was assessed using multivariate logistic regression analysis.
Results
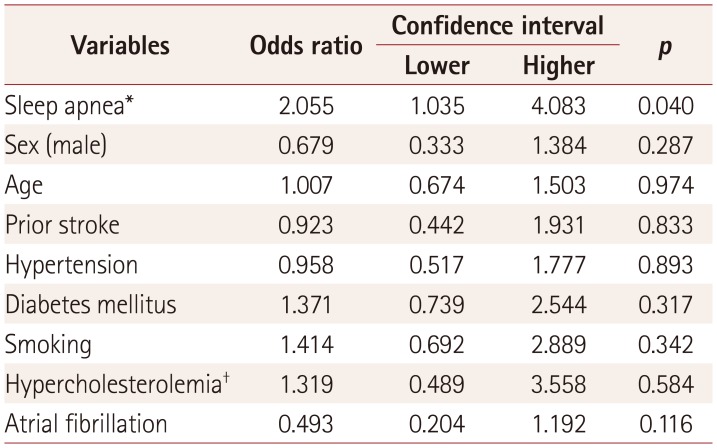
We identified 260 subjects with acute ischemic strokes with a definite onset time, of which 25.8% were WUS. The presence of preexisting witnessed or self-recognized sleep apnea was the only risk factor for WUS (adjusted odds ratio=2.055, 95% confidence interval=1.035–4.083, p=0.040).
Conclusions
Preexisting symptoms suggestive of OSA were associated with the occurrence of WUS. This suggests that OSA contributes to ischemic stroke not only as a predisposing risk factor but also as a triggering factor. Treating OSA might therefore be beneficial in preventing stroke, particularly that occurring during sleep.
Obstructive sleep apnea (OSA) is a recently identified risk factor for stroke, which is important since the condition is both highly prevalent and treatable. The recent revised stroke prevention guideline provided new recommendations for secondary stroke prevention as well as commenting on sleep apnea.1 However, it remains unclear whether the relationship between OSA and stroke is causative or merely a correlation. The association between OSA and stroke is often explained by the sharing of common comorbid conditions such as hypertension, diabetes mellitus, dyslipidemia, obesity, and atrial fibrillation, which are wellestablished stroke risk factors. Although more recent studies showed that OSA is an independent risk factor for stroke,23 these studies were not designed to identify the pathophysiologic mechanism linking OSA to stroke. Moreover, whether treating OSA decreases the risk of incident stroke also needs remains to be clarified.4
If OSA is a direct triggering factor for stroke, stroke might occur more frequently during sleep in people with OSA. Stroke occurring during sleep or wake-up stroke (WUS) reportedly constitute 13.5–38.9% of cases of ischemic stroke.5678910111213 Patients who wake up with stroke symptoms are often not eligible for thrombolytic therapy, since administering IV tissue plasminogen activator is restricted to 4.5 hours following the onset of stroke symptoms. Some studies found that WUS patients had a greater initial stroke severity and were more likely to experience a poor outcome,59 while many studies have found that risk-factor profiles do not differ significantly between WUS and non-WUS. There are several reports of dyslipidemia being more frequent in WUS.131415 Lower blood pressure was suggested as a risk factor for WUS,11 but this finding has not been replicated in other studies. Four12151617 of six111213151617 studies that assessed OSA as a stroke risk factor found that habitual snoring or a higher apnea-hypopnea index in poststroke polysomnography (PSG) was more frequent among WUS patients (Supplementary Table 1 in the online-only Data Supplement). However, poststroke PSG cannot be used to diagnose preexisting OSA due to the high prevalence of new-onset OSA following acute stroke. Based on the above-mentioned considerations, the present study examined whether preexisting symptoms of OSA are associated with the occurrence of WUS.
We consecutively evaluated adult patients who were admitted with acute ischemic stroke in Gangwon Comprehensive Stroke Center (CSC) in Kangwon National University Hospital between December 1, 2013 and April 30, 2015. Gangwon CSC is 1 of 11 regional CSCs in South Korea, and it covers the Gangwon province. This center was established in 2009 with support from the Ministry of Health and Welfare.
We included patients with acute ischemic stroke presenting via the emergency room (ER) within 7 days from the stroke onset. Patients were excluded if they had experienced a transient ischemic attack and hemorrhagic stroke. We also excluded those in whom the ischemic stroke occurred during hospitalization or if the patient was admitted via an outpatient clinic, since the intake interview could be standardized only when the patient initially presented to the ER. We used a standardized protocol to collect clinical information associated with stroke, laboratory findings, and preexisting symptoms of OSA. Patients or their caregivers were asked to provide a careful description of the situation and timing of stroke onset. The strokes were classified into WUS and non-WUS based on the time of stroke onset, where WUS was defined as the symptoms being recognized upon awakening by a witness or by the patient, and non-WUS was defined as the symptoms being recognized while performing daily activities. The strokes were categorized into the following subtypes using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, other determined etiology, and undetermined etiology. The routine workup for acute ischemic stroke was performed using brain CT, MRI, carotid ultrasonography, and transthoracic echocardiography. Selected patients underwent 24-hour Holter monitoring and transesophageal echocardiography when embolic infarction was strongly suggested and the source could not be found using the aforementioned routine tests.
The presence of OSA symptoms prior to the stroke was assessed by applying the Korean version of the Berlin Questionnaire18 to either the patient or caregiver. All questionnaires were administered by a single interviewer affiliated to Gangwon CSC while being blinded to clinical information, including the stroke onset time. This questionnaire comprises 11 questions grouped into 3 categories. Category 1 consists of five questions covering snoring, witnessed or self-recognized apnea, and the frequency of such events. Category 2 consists of four questions on daytime sleepiness; we modified the question “Have you ever fallen asleep while driving?” to “Have you had trouble staying awake while driving, eating meals, or engaging in social activity?” because most of the patients answered “I don't drive” in our pilot study. Category 3 consists of two questions concerning blood pressure and body mass index (BMI). Patients were considered to be at a high risk of sleep apnea when they conformed with at least two symptom categories.19
Statistical analysis was used to compare the demographic and clinical variables between WUS and non-WUS patients. The chi-square test or Fisher's exact test was used for categorical variables, while the independent t-test was used for continuous variables including age, cholesterol, and BMI. Multivariate logistic regression analysis was used to assess the association between witnessed or self-recognized sleep apnea and WUS. The model was adjusted for potential confounders including sex, age, history of prior stroke, hypertension, diabetes mellitus, smoking, hypercholesterolemia, and atrial fibrillation. We did not include obesity in the model due to the presence of a prior strong correlation with sleep apnea and BMI not differing significantly between WUS and non-WUS patients. For multivariate analysis, age was categorized to three groups of <60, 60–75, and >75 years, while hypercholesterolemia was considered to be present when total cholesterol was higher than 240 mg/dL.
The Statistical Package for Social Sciences (version 21 for Windows, IBM Corp., Armonk, NY, USA) was used for the statistical analyses. All reported p values are two-tailed, with p<0.05 defining the presence of statistical significance.
The study was approved by the Institutional Review Board of Kangwon National University Hospital (KNUH-2016-03-007-001).
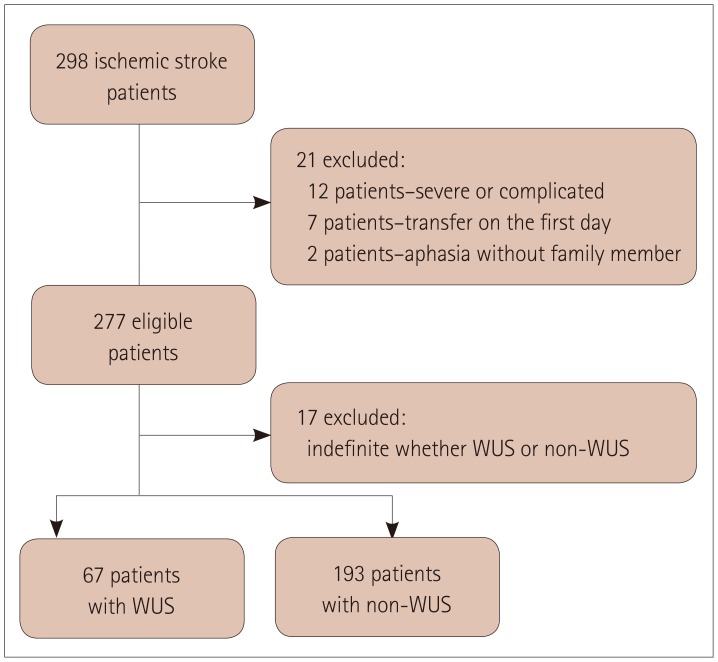
In total, 298 patients with acute ischemic stroke met the inclusion criteria. We could not obtain answered questionnaires for 21 patients because 12 had severe medical complications, 7 were transferred to other hospitals on the day of admission, and 2 patients had severe aphasia and no caregiver. Seventeen of the remaining 277 patients were excluded because the stroke onset could not be categorized into either WUS or non-WUS. Finally 260 patients were included in the final analysis: 67 (25.8%) in the WUS group and 193 (74.2%) in the non-WUS group (Fig. 1). The informants for the Berlin Questionnaire were the patients in 81 (31.2%) cases, cosleepers in 67 (25.8%) cases, and other family members in 112 (43.1%) cases. There were positive replies to Category 1 questions on snoring and apnea in 83 (31.9%) patients, to Category 2 questions on excessive daytime sleepiness in 21 (8.1%) patients, and to Category 3 questions on obesity and hypertension in 150 (57.7%) patients. Overall, 59 (22.7%) patients were considered at a high risk of sleep apnea based on the number of positive signs/symptoms in the different categories.
The clinical characteristics of the WUS and non-WUS patients are compared in Table 1. Witnessed or self-recognized sleep apnea was significantly more common in WUS patients than in non-WUS patients (28.3% versus 16.6%, p=0.036). However, the snoring and risk categorization for sleep apnea based on the Berlin Questionnaire did not differ significantly between these two groups. Atrial fibrillation was marginally more frequent in non-WUS than in WUS (20.7% versus 10.4%, p=0.060). The National Institute of Health Stroke Scale (NIHSS) scores at admission were more severe in WUS than in non-WUS (median=4 [interquartile range=2–9] versus 2 [1–4.5], p=0.013). None of the other demographic or stroke risk factors differed significantly between the two groups. Multivariate analysis confirmed that only sleep apnea was significantly associated with WUS (odds ratio=2.055, 95% confidence interval=1.035– 4.083, p=0.040) (Table 2).
We found that sleep apnea prior to stroke was associated with WUS. This suggests that OSA not only influences chronic changes that predispose patients to stroke but also produces more acute changes directly resulting in stroke. Treating OSA might therefore be beneficial in preventing stroke, even in elderly patients with long-standing disease. Plausible mechanisms for OSA-associated ischemic stroke include the immediate effect of hypoxia, decreased cerebral blood flow, and the paradoxical embolism induced by apnea events, plaque disruption associated with vibration, and hypercoagulability that have been noted in OSA patients.2021
Our finding that the incidence of stroke while awake was higher in those with atrial fibrillation supports the traditional idea that embolic stroke is more likely to occur while active.22 In agreement with a few previous studies, the initial NIHSS score at admission was higher in WUS than in non-WUS. This could be due to not only the delay in detecting and treating stroke, but also being in a worse condition such as decreased systemic blood pressure or lower cerebral blood flow, or hypercoagulability during sleep.232425 The association between OSA and WUS is more meaningful in this regard.
Our study was subject to some limitations. The study had a cross-sectional design and the questionnaire was the only way to identify the presence of OSA symptoms preceding the onset of stroke, because poststroke PSG does not indicate preexisting OSA. Classifying a high versus a low risk of sleep apnea based on the category scores of the Berlin Questionnaire was reported to have low validity in the elderly, mainly due to hypertension being highly prevalent and the difficulty of assessing sleepiness in the elderly. Simply using Category 1 on snoring and apnea was more valid than using a high/low classification,26 supporting our results that WUS was associated with the presence of apnea rather than a high-risk classification. Witnessed sleep apnea had a high specificity and low sensitivity for significant OSA confirmed by PSG, whereas snoring had a high sensitivity and relatively low specificity.27
Despite the validity issues, the prevalence of OSA symptoms in our study was comparable to the prevalence of PSG-confirmed OSA in a previous study performed in Korea.28 While our study was conducted in a single center, Kangwon National University Hospital is a teaching hospital that provides advanced specialty care and has a large catchment area that encompasses the province with easy access for patients. We therefore consider the data from a model to be less biased. However, the findings of this study need to be confirmed in larger-scale prospective cohort studies that apply PSG to evaluate underlying OSA and long-term follow-up in order to detect the occurrence of stroke and stroke onset time.
In conclusion, preexisting symptoms of OSA were associated with the occurrence of WUS. This result has highly practical implications for preventing WUS and provides insight into the mechanism underlying how OSA influences the incidence of stroke.
Acknowledgements
This study was supported by 2016 Research Grant from Kangwon National University (No. 520160371).
We are indebted to Hye-Yeon Jang, RN of Gangwon CSC for interviewing patients or caregivers, to Yeong-Kwon Park, PhD of Gangwon CSC for collecting data and managing database, and Rachel Maurer, MD of UIHC Sleep Disorders Center for preparation of manuscript.
References
1. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236. PMID: 24788967.
2. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353:2034–2041. PMID: 16282178.

3. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010; 182:269–277. PMID: 20339144.

4. Kim Y, Koo YS, Lee HY, Lee SY. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PLoS One. 2016; 11:e0146317. PMID: 26731604.

5. Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, et al. Population-based study of wake-up strokes. Neurology. 2011; 76:1662–1667. PMID: 21555734.

6. Spengos K, Tsivgoulis G, Manios E, Synetou M, Vassilopoulou S, Zakopoulos N, et al. Stroke etiology is associated with symptom onset during sleep. Sleep. 2005; 28:233–238. PMID: 16171248.

7. Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD. Registry of the Canadian Stroke Network. Outcome after stroke upon awakening. Can J Neurol Sci. 2005; 32:232–236. PMID: 16018160.

8. Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, et al. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis. 2010; 29:336–342. PMID: 20130399.

9. Kim BJ, Lee SH, Shin CW, Ryu WS, Kim CK, Yoon BW. Ischemic stroke during sleep: its association with worse early functional outcome. Stroke. 2011; 42:1901–1906. PMID: 21546480.
10. Moradiya Y, Janjua N. Presentation and outcomes of “wake-up strokes” in a large randomized stroke trial: analysis of data from the International Stroke Trial. J Stroke Cerebrovasc Dis. 2013; 22:e286–e292. PMID: 22939198.

11. Bassetti C, Aldrich M. Night time versus daytime transient ischaemic attack and ischaemic stroke: a prospective study of 110 patients. J Neurol Neurosurg Psychiatry. 1999; 67:463–467. PMID: 10486392.

12. Hsieh SW, Lai CL, Liu CK, Hsieh CF, Hsu CY. Obstructive sleep apnea linked to wake-up strokes. J Neurol. 2012; 259:1433–1439. PMID: 22215237.

13. Tanimoto A, Mehndiratta P, Koo BB. Characteristics of wake-up stroke. J Stroke Cerebrovasc Dis. 2014; 23:1296–1299. PMID: 24361192.

14. Kim TJ, Ko SB, Jeong HG, Lee JS, Kim CK, Kim Y, et al. Nocturnal desaturation in the stroke unit is associated with wake-up ischemic stroke. Stroke. 2016; 47:1748–1753. PMID: 27222527.

15. Šiarnik P, Kollár B, Čarnická Z, Šurda P, Klobučníková K, Sýkora M, et al. Association of sleep disordered breathing with wake-up acute ischemic stroke: a full polysomnographic study. J Clin Sleep Med. 2016; 12:549–554. PMID: 26612509.

16. Martínez García MA, Galiano Blancart R, Cabero Salt L, Soler Cataluña JJ, Escamilla T, Román Sánchez P. [Prevalence of sleep-disordered breathing in patients with acute ischemic stroke: influence of onset time of stroke]. Arch Bronconeumol. 2004; 40:196–202. PMID: 15117618.

17. Palomäki H, Partinen M, Juvela S, Kaste M. Snoring as a risk factor for sleep-related brain infarction. Stroke. 1989; 20:1311–1315. PMID: 2799862.

18. Kang K, Park KS, Kim JE, Kim SW, Kim YT, Kim JS, et al. Usefulness of the Berlin Questionnaire to identify patients at high risk for obstructive sleep apnea: a population-based door-to-door study. Sleep Breath. 2013; 17:803–810. PMID: 23054593.

19. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999; 131:485–491. PMID: 10507956.

20. Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009; 136:1668–1677. PMID: 19995768.

21. Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004; 3:333–342. PMID: 15157848.

22. Chaturvedi S, Adams HP Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke. 1999; 30:1792–1795. PMID: 10471425.

23. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003; 4:649–661. PMID: 12894240.

24. Zoccoli G, Walker AM, Lenzi P, Franzini C. The cerebral circulation during sleep: regulation mechanisms and functional implications. Sleep Med Rev. 2002; 6:443–455. PMID: 12505477.

25. Bridges AB, McLaren M, Scott NA, Pringle TH, McNeill GP, Belch JJ. Circadian variation of tissue plasminogen activator and its inhibitor, von Willebrand factor antigen, and prostacyclin stimulating factor in men with ischaemic heart disease. Br Heart J. 1993; 69:121–124. PMID: 8435236.

26. Sforza E, Chouchou F, Pichot V, Herrmann F, Barthélémy JC, Roche F. Is the Berlin questionnaire a useful tool to diagnose obstructive sleep apnea in the elderly? Sleep Med. 2011; 12:142–146. PMID: 21227749.

27. Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest. 1991; 99:600–608. PMID: 1995215.

28. Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004; 170:1108–1113. PMID: 15347562.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.2.174
Table 1
Clinical characteristics according to the time of stroke onset
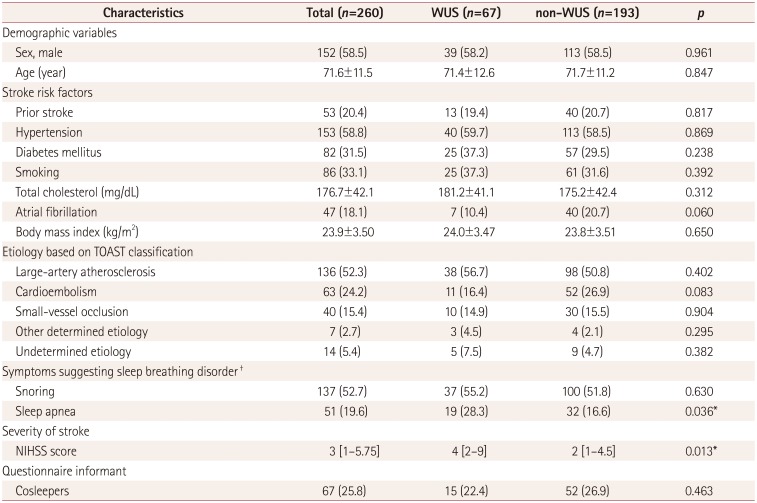
Data are mean±standard deviation, n (%), or median [interquartile range] values.
*p<0.05, †Snoring, at least once a month; sleep apnea, witnessed or self-recognized sleep apnea at least once a month.
NIHSS: National Institute of Health Stroke Scale, TOAST: Trial of Org 10172 in Acute Stroke Treatment, WUS: wake-up stroke.
Table 2
Odds ratios and confidence intervals for wake-up stroke in the multivariate analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download