Abstract
Background and Purpose
The objective of this study was to determine if the MOXO visual- and vocal-distractors-based continuous performance test distinguishes patients with attention deficit hyperactivity disorder (ADHD) and neurofibromatosis type 1 (NF1) from those without NF1.
Methods
Thirty-five patients (16 males; mean age 9.91 years) attending a multidisciplinary NF1 clinic completed the MOXO test. The findings were compared to 532 healthy age-matched standardized control subjects (285 males) without ADHD.
Results
The overall performance in the MOXO text was significantly worse in the NF1 group than in controls (p<0.01), but no group-specific pattern was identified. Impulsivity and hyperactivity were significantly more prominent in males than females (p<0.01). Compared to controls, the NF1 group exhibited significantly more failures to respond to target stimuli in the presence of visual distractors.
Neurofibromatosis type 1 (NF1) is a genetic disorder with an autosomal dominant pattern of inheritance. It is characterized clinically by multiple café-au-lait macules, axillary and/or inguinal freckling, iris Lisch nodules, and tumors of the nervous system such as neurofibromas and optic pathway gliomas.1 The disease is caused by mutations in NFI that encodes for neurofibromin, a GTPase-activating protein for Ras. Loss of function of neurofibromin leads to an overall increase in the active GTP-Ras complex and a consequent risk of tumor formation.2
NF1 is often associated with learning, cognitive, and behavioral disabilities which account for a significant proportion of the disease-related morbidity and can have a profound impact on quality of life.3 The incidence of attention deficit hyperactivity disorder (ADHD) in patients with NF1 ranges between 33% and 49.5%,4 with no sex difference. According to the Centers for Disease Control and Prevention (CDC) report from 2011–2013, the percentage of ADHD ever diagnosed among children age 4–17 was 11.5%, and by sex: 13.3% in males and 5.6% in females.5
The ADHD-like symptoms in NF1 appear to involve complex impairments in cognitive processes, visuospatial function, and executive function that may operate through mechanisms that probably differ from those responsible for ADHD in individuals without NF1.6 Researchers have suggested that impaired reactivity to visual signals plays a role.7
The aim of the present study was to determine if the MOXO test for ADHD (Neurotech Solutions, Nes Ziona, Israel), which is a visual- and vocal-distractors-based continuous performance test, can distinguish between patients with ADHD with and without NF1, and if attention deficits in patients with NF1 are related to abnormal visual responses.
The study was approved by our Local Ethics Committee (approval number: 0192-11-RMC).
Thirty-five children and adolescents aged 6–17 years with NF1 were recruited for the study from the multidisciplinary NF1 clinic of a large pediatric tertiary referral, university-affiliated medical center. The diagnosis of NF1 was based on the 1988 guidelines of the National Institutes of Health.8 Parents provided written informed consent for their children/adolescents to participate in the study, and the children/adolescents provided verbal assent.
Background data on the children/adolescents were collected from computerized patient medical files. In addition, parents and teachers completed the Conners Comprehensive Behavior Rating Scales,9 which measures learning problems and executive functioning, and the questionnaire for ADHD in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). All 35 children completed the MOXO continuous performance test for ADHD (Neurotech Solutions), which is a standardized computerized test designed to diagnose the following four ADHD-related symptoms: inattention, timing, impulsivity, and hyperactivity.1011 The test was administered in a quiet room in the pediatric neurology clinic. The results were analyzed, together with the parent and teacher reports, and the conclusions and implications of the findings were presented to the patients and parents at the next clinic visit.
For purposes of the study, the results of the patients with NF1 (experimental group) were compared with those of an age-matched standardized group of children without NF1 who participated in the original studies validating the MOXO and were found not to have ADHD (control group).1011 The conditions of administration of the MOXO were identical in both groups.
In brief, subjects are seated in front of a computer screen and presented with a series of stimuli, some of which are designated as target stimuli. Subjects are asked to respond as quickly as possible to the target stimuli by pressing the space bar on the keyboard. In each trial, a stimulus (target or nontarget) is presented for 500, 1,000, or 4,000 milliseconds. The target remains on the screen for the full duration of the designation time, whether or not a response was given. This is followed by a rest period of the same duration. In this manner, it is possible to determine both the timing of the response, if it occurred (during stimulus presentation or the rest period), as well as its accuracy. Participants are instructed to press the space bar once and only once when they see the target, to not press any key other than the space bar, and to ignore any stimulus other than the target stimulus.
The test includes eight distractors (neither target nor nontarget stimuli) that take the form of short video clips. Distractors may be visual, auditory, or both (e.g., an animated barking dog, the background sound of a barking dog, or an animated dog with background barking), and they are presented simultaneously with the target/nontarget stimuli. The number and type of distractors presented during the test vary, as follows: levels 1 and 8, no distractor; levels 2 and 3, simple and complex visual distractors only; levels 4 and 5, simple and complex auditory distractors only; and levels 6 and 7, combined simple visual and auditory distractors and complex combined distractors.12,13 The levels of the distractors are always presented in numerical order. The total test includes 8 trials and 262 targets, and total duration of the test is 15 min.
BMDP statistical software14 was used for the statistical analysis. Discrete variables were analyzed with Pearson's test, the chisquared test, or Fisher's exact test, as appropriate, and continuous variables were analyzed with one-way analysis of variance or the nonparametric Mann-Whitney U-test. A p value of ≤0.01 was considered statistically significant. The z-test was applied to each significance level.
The experimental group comprised 16 males and 19 females aged 9.91±3.72 years (mean±SD). Thirteen patients (37.2%) had a first-degree relative with NF1. All 35 children were attending mainstream schools and were considered to have a normal IQ according to the school records. The control group comprised 285 males and 247 females aged 10.14±2.75 years.
Significant differences were found for all four ADHD parameters evaluated by the MOXO test between the experimental and control groups (p<0.01). The z-scores for the experimental group are listed in Table 1. Analysis by sex showed that while the males performed better than the females in attention (correct responses to the targets, early or late) and timing (correct responses to the targets before the rest period), they exhibited worse scores for impulsivity and hyperactivity (responding to nontarget stimuli, failing to respond to target stimuli, and pressing keys other than the space bar, as presented in Table 2). Older children (those aged 13–18 years) showed a tendency toward higher impulsivity and hyperactivity than younger children (those aged 6–12 years), but the difference did not reach statistical significance (Fig. 1).
The errors of omission (failures to respond to target stimuli) in the absence of distractors (levels 1 and 8) did not differ over time (i.e., between the beginning and end of the test). The number of omission errors increased significantly in the younger group when simple visual distractors or combined visual and auditory distractors were presented relative to no distractor (p<0.01) (Table 3). The increase in the number of omission errors in the older group when combined distractors were present was not statistically significant (Table 3).
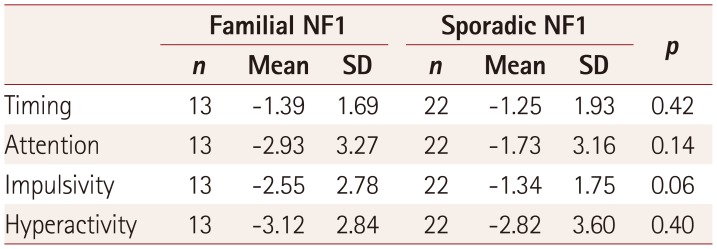
Patients with a family history of NF1 had more impulsivity than patients with sporadic NF1 (Table 4). None of the children with NF1 incorrectly responded to a non-target stimulus on the MOXO.
Parental responses on the DSM-IV questionnaire were available for 32 children. Two children (6%) had an attention deficit only and 15 (47%) had combined attention deficit and hyperactivity, with abnormal results found for 9 children (28%) on the Connors Parent Rating Scale. Teacher responses on the DSM-IV questionnaire were available for 26 children. Four children (15%) had an attention deficit only and 6 (23%) had combined attention deficit and hyperactivity, with abnormal results found for 6 children (23%) on the Conners Teacher Rating Scale.
The present study has shown that patients with NF1 produce abnormal results on the MOXO test, but that the test cannot distinguish them from patients with ADHD without NF1. However, the test identified important sex differences in this population, with males tending to have more impulsivity and hyperactivity than females but better attention and timing. This contrasts with reports of there being more overall ADHD symptoms in males than females,15 and males having more emotional and prosocial behavior problems.16 A previous study using the Strengths and Difficulties Questionnaire produced similar results.17 Importantly, the MOXO test revealed impairment in reactivity in the presence of visual distractors in children and adolescents with NF1.
Several studies suggest that the cognitive, motor, learning, and social problems often identified in patients with NF1 are not a distinct comorbidity of the disease but rather a direct consequence of NF1 pathology.18 Koth et al.19 compared the ADHD status of children with NF1 to that of their healthy siblings and biological parents. They found a significant connection between NF1 and ADHD, suggesting that ADHD occurs as a component of the underlying disease. While the mechanism has not been established, researchers seeking a candidate gene for ADHD are focusing on genes involved in cellular growth.20 Accordingly, imaging studies have found cognitive dysfunction, learning disabilities, and attention deficit to be associated with T2 hyperintensities in the thalamus and other brain areas in children with NF1 but not in children with ADHD.2122
These findings are consistent with the high rate of omission errors in the presence of visual distractors in our children/adolescents with NF1. In a related study, Michael et al.23 asked patients with NF1 to locate a target as quickly and as accurately as possible while ignoring any potential distractor that could appear before, simultaneously with, or after the target. They found that during attentive processing, patients with NF1 tended to overreact to or overinspect visual signals occurring outside the focus of attention. Even though we found an increase in omission errors rather than commission errors in our test, it seems that the visuospatial analysis abilities of NF1 patients are deficient. Other authors have suggested that the poor performance of children with NF1 on visuospatial tasks indicates a deficiency in information processing in the early visual areas.24 This notion is supported by a functional magnetic resonance imaging (MRI) study of both children and adults with NF1 finding deficient activation of the early visual cortex pathways in response to low-level visual stimulation compared to healthy controls, which was not ameliorated with age.25
Ribeiro et al.26 used encephalography/event-related potentials to evaluate the neural mechanism involved in impaired visual responses in children and adolescents with NF1. Abnormalities were identified in later stages of visual processing in addition to enhanced alpha oscillations, which appears to point to deficits in basic sensory processing. Although the cause of the abnormal alpha rhythm in NF1 was unclear, its possible association with thalamic dysfunction is supported by the known strategic role that the thalamus plays in the generation of normal alpha rhythms27 and the abnormal structure and metabolism of the thalamus in patients with NF1.212228 In the present study, the age-related differences in the visuospatial performance in the MOXO trials could have been due to a maturation delay, as previously suggested in otherwise healthy patients with ADHD,2930 or it may be related to previously reported reductions over time in T2 hyperintensities in the thalamus and other brain areas.22
In conclusion, patients with NF1 and characteristics of ADHD cannot be distinguished from otherwise healthy children with ADHD using the MOXO test. However, this test does reveal the significant impairment in response to visual distractors, which improves slightly with age. This might constitute part of the attention problem experienced by patients with NF1.
Further investigations are needed in larger numbers of NF1 patients and including comparing these results with MRI findings.
References
1. Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009; 123:124–133. PMID: 19117870.

3. Acosta MT, Bearden CE, Castellanos FX, Cutting L, Elgersma Y, Gioia G, et al. The Learning Disabilities Network (LeaDNet): using neurofibromatosis type 1 (NF1) as a paradigm for translational research. Am J Med Genet A. 2012; 158A:2225–2232. PMID: 22821737.

4. Payne JM, Hyman SL, Shores EA, North KN. Assessment of executive function and attention in children with neurofibromatosis type 1: relationships between cognitive measures and real-world behavior. Child Neuropsychol. 2011; 17:313–329. PMID: 21347908.

5. Centers for Disease Control and Prevention (US). Attention Deficit Hyperactivity Disorder (ADHD) [Internet]. Atlanta (GA): CDC/National Center for Health Statistics;2017. cited 2018 Feb. Available from:https://www.cdc.gov/nchs/fastats/adhd.htm.
6. Miguel CS, Chaim-Avancini TM, Silva MA, Louzã MR. Neurofibromatosis type 1 and attention deficit hyperactivity disorder: a case study and literature review. Neuropsychiatr Dis Treat. 2015; 11:815–821. PMID: 25848279.

7. Descheemaeker MJ, Plasschaert E, Frijns JP, Legius E. Neuropsychological profile in adults with neurofibromatosis type 1 compared to a control group. J Intellect Disabil Res. 2013; 57:874–886. PMID: 23095048.

8. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988; 45:575–578. PMID: 3128965.
9. Conners CK. Conners Comprehensive Behavior Rating Scales™. New York: Multi-Health Systems Inc.;2008.
10. Berger I, Goldzweig G. Objective measures of attention-deficit/hyperactivity disorder: a pilot study. Isr Med Assoc J. 2010; 12:531–535. PMID: 21287795.
11. Berger I, Slobodin O, Cassuto H. Usefulness and validity of continuous performance tests in the diagnosis of attention-deficit hyperactivity disorder children. Arch Clin Neuropsychol. 2017; 32:81–93. PMID: 28122767.
12. Berger I, Cassuto H. The effect of environmental distractors incorporation into a CPT on sustained attention and ADHD diagnosis among adolescents. J Neurosci Methods. 2014; 222:62–68. PMID: 24211249.

13. Cassuto H, Ben-Simon A, Berger I. Using environmental distractors in the diagnosis of ADHD. Front Hum Neurosci. 2013; 7:805. PMID: 24319423.

14. Dixon WJ. BMPD Statistical Software Manual. Berkeley: University of California Press;1990.
15. Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010; 49:217–228. PMID: 20410711.

16. Nøvik TS, Hervas A, Ralston SJ, Dalsgaard S, Rodrigues Pereira R, Lorenzo MJ. ADORE Study Group. Influence of gender on attention-deficit/ hyperactivity disorder in Europe--ADORE. Eur Child Adolesc Psychiatry. 2006; 15(Suppl 1):I15–I24. PMID: 17177011.
17. Cohen R, Steinberg T, Kornreich L, Aharoni S, Halevy A, Shuper A. Brain imaging findings and social/emotional problems in Israeli children with neurofibromatosis type 1. Eur J Pediatr. 2015; 174:199–203. PMID: 25027832.

18. Lidzba K, Granström S, Lindenau J, Mautner VF. The adverse influence of attention-deficit disorder with or without hyperactivity on cognition in neurofibromatosis type 1. Dev Med Child Neurol. 2012; 54:892–897. PMID: 22881119.

19. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000; 6:185–194. PMID: 11402396.

20. Huijbregts S. Cognitive-behavioral phenotype or comorbid disorder? The case of attention-deficit-hyperactivity disorder in neurofibromatosis type 1. Dev Med Child Neurol. 2012; 54:873–874. PMID: 22881385.

21. Denckla MB, Hofman K, Mazzocco MM, Melhem E, Reiss AL, Bryan RN, et al. Relationship between T2-weighted hyperintensities (unidentified bright objects) and lower IQs in children with neurofibromatosis-1. Am J Med Genet. 1996; 67:98–102. PMID: 8678124.

22. Hyman SL, Gill DS, Shores EA, Steinberg A, North KN. T2 hyperintensities in children with neurofibromatosis type 1 and their relationship to cognitive functioning. J Neurol Neurosurg Psychiatry. 2007; 78:1088–1091. PMID: 17299016.

23. Michael GA, Garcia S, Herbillon V, Lion-François L. Reactivity to visual signals in neurofibromatosis type 1: is everything ok? Neuropsychology. 2014; 28:423–428. PMID: 24274026.

24. Clements-Stephens AM, Rimrodt SL, Gaur P, Cutting LE. Visuospatial processing in children with neurofibromatosis type 1. Neuropsychologia. 2008; 46:690–697. PMID: 17988695.

25. Violante IR, Ribeiro MJ, Cunha G, Bernardino I, Duarte JV, Ramos F, et al. Abnormal brain activation in neurofibromatosis type 1: a link between visual processing and the default mode network. PLoS One. 2012; 7:e38785. PMID: 22723888.

26. Ribeiro MJ, d'Almeida OC, Ramos F, Saraiva J, Silva ED, Castelo-Branco M. Abnormal late visual responses and alpha oscillations in neurofibromatosis type 1: a link to visual and attention deficits. J Neurodev Disord. 2014; 6:4. PMID: 24559228.

27. Hughes SW, Crunelli V. Just a phase they're going through: the complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol. 2007; 64:3–17. PMID: 17000018.
28. Payne JM, Moharir MD, Webster R, North KN. Brain structure and function in neurofibromatosis type 1: current concepts and future directions. J Neurol Neurosurg Psychiatry. 2010; 81:304–309. PMID: 20185469.

29. Berger I, Slobodin O, Aboud M, Melamed J, Cassuto H. Maturational delay in ADHD: evidence from CPT. Front Hum Neurosci. 2013; 7:691. PMID: 24298243.

30. Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012; 72:191–197. PMID: 22418014.

Fig. 1
Percentage of neurofibromatosis type 1 patients with z-scores of ≤1.65 according to age. A: attention, H: hyperactivity, I: impulsivity, T: timing.
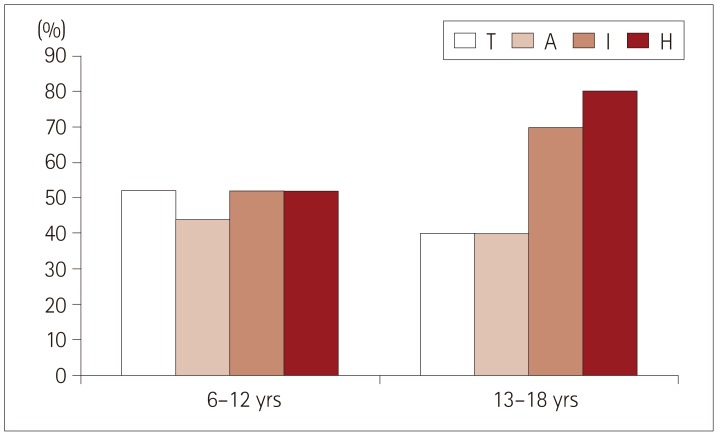
Table 1
Mean z-scores for attention deficit hyperactivity disorder parameters in patients with neurofibromatosis type 1 compared to normal values
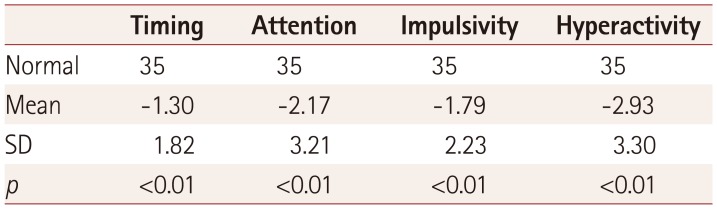
| Timing | Attention | Impulsivity | Hyperactivity | |
|---|---|---|---|---|
| Normal | 35 | 35 | 35 | 35 |
| Mean | −1.30 | −2.17 | −1.79 | −2.93 |
| SD | 1.82 | 3.21 | 2.23 | 3.30 |
| p | <0.01 | <0.01 | <0.01 | <0.01 |
Table 2
Neurofibromatosis type 1 score for each index according to sex
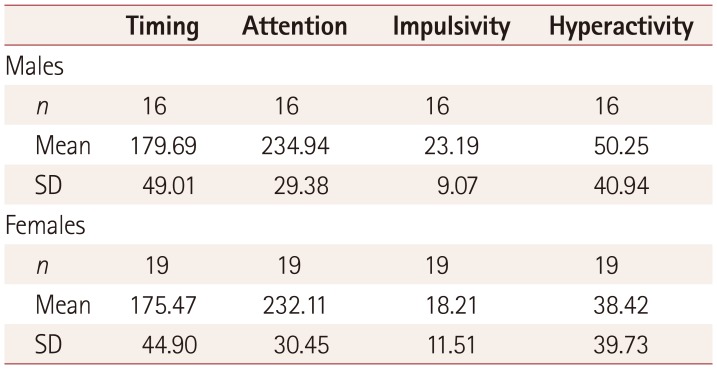
| Timing | Attention | Impulsivity | Hyperactivity | |
|---|---|---|---|---|
| Males | ||||
| n | 16 | 16 | 16 | 16 |
| Mean | 179.69 | 234.94 | 23.19 | 50.25 |
| SD | 49.01 | 29.38 | 9.07 | 40.94 |
| Females | ||||
| n | 19 | 19 | 19 | 19 |
| Mean | 175.47 | 232.11 | 18.21 | 38.42 |
| SD | 44.90 | 30.45 | 11.51 | 39.73 |
Table 3
Differences in omission errors between children and adolescents with NF1 and ADHD and a standardized control group of children without ADHD1011
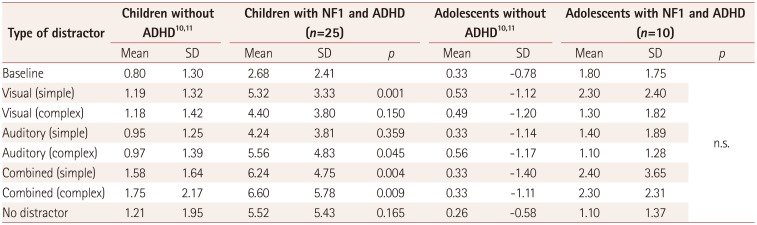
| Type of distractor | Children without ADHD1011 | Children with NF1 and ADHD (n=25) | Adolescents without ADHD1011 | Adolescents with NF1 and ADHD (n=10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| Baseline | 0.80 | 1.30 | 2.68 | 2.41 | 0.33 | −0.78 | 1.80 | 1.75 | n.s. | |
| Visual (simple) | 1.19 | 1.32 | 5.32 | 3.33 | 0.001 | 0.53 | −1.12 | 2.30 | 2.40 | |
| Visual (complex) | 1.18 | 1.42 | 4.40 | 3.80 | 0.150 | 0.49 | −1.20 | 1.30 | 1.82 | |
| Auditory (simple) | 0.95 | 1.25 | 4.24 | 3.81 | 0.359 | 0.33 | −1.14 | 1.40 | 1.89 | |
| Auditory (complex) | 0.97 | 1.39 | 5.56 | 4.83 | 0.045 | 0.56 | −1.17 | 1.10 | 1.28 | |
| Combined (simple) | 1.58 | 1.64 | 6.24 | 4.75 | 0.004 | 0.33 | −1.40 | 2.40 | 3.65 | |
| Combined (complex) | 1.75 | 2.17 | 6.60 | 5.78 | 0.009 | 0.33 | −1.11 | 2.30 | 2.31 | |
| No distractor | 1.21 | 1.95 | 5.52 | 5.43 | 0.165 | 0.26 | −0.58 | 1.10 | 1.37 | |
Table 4
z-scores for attention deficit hyperactivity disorder parameters according to type of NF1




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download