Abstract
Background and Purpose
Neurolymphomatosis is a rare manifestation of hematological malignancy and is characterized by direct infiltration of the peripheral nervous system. The objective of this study was to identify the clinical and electrophysiological features of neurolymphomatosis.
Methods
We retrospectively analyzed the medical records of 13 patients with neurolymphomatosis. Seven (54%) of the patients were men, and the median age at symptom onset was 60.0 years.
Results
The most common type of underlying malignancy was diffuse large B-cell lymphoma (69%). Twelve patients had painful asymmetric neuropathies. The median survival time after diagnosis was 7 months, and 12 patients died during the study period. Thirty-eight motor nerve conduction studies (NCSs) were performed in the affected nerves. Ten and 28 motor nerves were classified into the conduction-block and simple-axon-degeneration groups, respectively. The median time interval between symptom onset and the NCS was significantly shorter in the conduction-block group than in the simple-axon-degeneration group (p=0.032). However, no significant differences in the motor nerve conduction velocities, terminal latencies, and distal compound muscle action potential amplitudes were identified between the conduction-block and simple-axon-degeneration groups. The conduction-block group showed excessive temporal dispersion in only five of the ten NCSs (50%). Follow-up NCSs revealed that partial conduction blocks had changed into axonal degeneration patterns.
Neurolymphomatosis is an extranodal manifestation of hematological malignancy and is characterized by direct infiltration of the peripheral nervous system. Most cases of neurolymphomatosis are associated with non-Hodgkin's lymphoma, especially diffuse large B-cell lymphoma.1 Neurolymphomatosis is generally thought to be an extremely rare disease; however, in one autopsy series neurolymphomatosis occurred in about 40% of patients with non-Hodgkin's lymphoma.2 The clinical presentation of neurolymphomatosis is highly variable. The most common clinical feature is painful polyradiculopathy or multiple mononeuropathies, followed by painless neuropathy, cranial neuropathy, and mononeuropathy. Neurolymphomatosis can develop both as a recurrent disease and as the first clinical presentation of hematological malignancy.34 Additionally, hematological malignancy can be associated with not only neurolymphomatosis but also other neuropathies including paraneoplastic neuropathy, autoimmune neuropathy, herpes zoster neuropathy, and chemotherapy-induced polyneuropathy. 56 This clinical heterogeneity can hinder and delay the diagnosis of neurolymphomatosis and may lead to a poor prognosis.7
Nerve biopsy is the gold standard for diagnosing neurolymphomatosis, but this approach inevitably results in neurological deficits. Magnetic resonance imaging (MRI) and 18F-2-fluoro-2-deoxy-D-glucose–positron-emission tomography/computed tomography (FDG-PET/CT) are noninvasive imaging tools that can indirectly demonstrate lymphomatous infiltration of the peripheral nerves and play key roles in the diagnosis of neurolymphomatosis.189 A nerve conduction study (NCS) is a basic and important modality for evaluating peripheral neuropathy. NCSs usually reveal axonal degeneration in patients with neurolymphomatosis, but may occasionally reveal a partial conduction block. Such a block is a typical nerve conduction finding of segmental demyelination,101112 but its meaning is unclear in neurolymphomatosis.
To understand the clinical manifestations of neurolymphomatosis, we investigated the clinical and electrophysiological features of this disease, including the characteristics of neurolymphomatosis-related partial conduction block.
We enrolled patients with neurolymphomatosis who visited Severance Hospital, College of Medicine, Yonsei University and Mokdong Hospital, College of Medicine, Ewha Womans University from January 2004 to May 2017. Patients with the following conditions were identified from medical records: 1) pathological diagnosis of hematological malignancy, 2) clinical evidence of peripheral neuropathy, and 3) abnormal enlargement, enhancement, or FDG uptake of clinically affected neural structures on limb/plexus MRI and FDG-PET/CT scans. We then excluded patients with underlying peripheral neuropathies caused by diabetes mellitus, alcohol, autoimmune diseases, and heavy metals. Finally, 13 patients with neurolymphomatosis who met the above criteria were selected, of whom 11 and 2 were evaluated at Severance Hospital and Mokdong Hospital, respectively. The present study was initiated after obtaining approval from our Institutional Review Board (IRB number: 4-2017-0386). The need for written informed consent was waived by the review board because this study had a retrospective design.
Clinical data were obtained retrospectively through the medical records of the patients. The following clinical information was collected: sex, age at symptom onset, affected nerve structures, pain, muscle weakness, sensory deficit, malignant cell type, and mortality. We additionally analyzed the effectiveness of various diagnostic modalities such as limb/plexus MRI, FDG-PET/CT, cerebrospinal fluid (CSF) cytology, and nerve biopsy.
The medical records revealed that NCSs were performed in all but one patient (patient 9). These electrophysiological studies were performed using standardized techniques with two electromyography systems: Cadwell Sierra Wave (Cadwell Laboratories, Kennewick, WA, USA) and Medelec Synergy (Oxford Instruments, Surrey, UK). Skin temperature was controlled to within the range of 32℃ to 36°C. Motor and sensory NCSs were performed with surface electrodes using the conventional technique as outlined by Oh.13 Terminal latencies, compound muscle action potentials (CMAPs), and motor nerve conduction velocities (NCVs) had been recorded from the median, ulnar, peroneal, and tibial nerves by the belly-tendon method. The sensory nerve action potentials and sensory NCVs had been recorded from the median, ulnar, and superficial peroneal and sural nerves.
A partial conduction block is defined as more than 50% decline of the negative-peak amplitude of the CMAP between distal and proximal stimulations for median and ulnar nerves and more than 60% decline of the negative-peak amplitude of the CMAP for peroneal and posterior tibial nerves, based on an American Association of Electrodiagnostic Medicine (AAEM) consensus report.14 Additionally, affected nerves were defined as testable terminal branches of nerves with abnormal enlargement, enhancement, or FDG uptake on limb/plexus MRI and FDG-PET/CT scans. We then classified the findings of the motor NCSs into the conduction-block and simple-axon-degeneration groups. The conduction-block group was defined as motor NCS findings of partial conduction block. The simple-axon-degeneration group was defined as motor NCSs that showed CMAPs <80% of the lower limit of normal without marked slowing of the conduction velocity (<60% of the normal mean) and marked prolongation of the distal latency (>150% of the normal mean).13
The Mann-Whitney test was used to compare the median time intervals between symptom onset and NCS findings in the conduction-block and simple-axon-degeneration groups. Differences were considered statistically significant at p<0.05. Statistical analyses were performed with SPSS (version 18.0, SPSS Inc., Chicago, IL, USA).
The clinical presentations of the 13 patients with neurolymphomatosis are summarized in Table 1. Seven (54%) of the patients were men, and the median age at symptom onset was 60.0 years [interquartile range (IQR)=41.0–66.0 years]. The median time interval between symptom onset and diagnosis was 65 days (IQR=26.0–102.5 days). Neurolymphomatosis was the first manifestation of hematological malignancy in four (31%) patients and the relapse of a known malignancy in nine (69%). The underlying malignancy was diffuse large B-cell lymphoma in nine (69%) patients, natural-killer/T-cell lymphoma in two (15%), T-cell acute lymphoblastic leukemia in one (8%), and a not-classified lymphoma in one (8%). Six (46%) and six (46%) patients had brain and cranial nerve involvement, respectively. Twelve (92%) patients had painful asymmetric neuropathies.
All patients received systemic chemotherapy, and four patients (patients 4, 6, 7, and 13) received intrathecal injections of methotrexate. Radiation therapy was performed in four patients (patients 3, 4, 11, and 12). Intravenous immunoglobulin therapy was performed in only one patient (patient 5), and no patient received plasmapheresis. The median survival time after diagnosis was 7 months (IQR=3.0–15.0 months), and 12 (92%) patients died during the study period. Patient 12 was alive at the last follow-up (5 months after diagnosis), but lymphoma had spread to the right facial nerve at 4 months after the diagnosis of neurolymphomatosis.
Neurolymphomatosis was diagnosed in all 13 patients based on FDG-PET/CT scans demonstrating increased linear or focal FDG uptake of the affected nerves (Supplementary Fig. 1 in the online-only Data Supplement). Limb/plexus MRI was performed in 12 patients, which revealed abnormal enlargement or enhancement in 10 (83%) patients. All patients had negative findings for autoimmune antibodies including antinuclear antibodies, anti-double-stranded DNA antibodies, antineutrophil cytoplasmic antibodies, and anti-Ro and anti-La antibodies. CSF cytology was performed in eight patients, which revealed malignant cells in three (patients 1, 3, and 9). A nerve biopsy was performed in three patients: patients 5 and 10 exhibited axonal degeneration with malignant cell infiltrates, while patient 8 showed only axonal degeneration (Supplementary Fig. 2 in the online-only Data Supplement).
The NCS findings were compatible with multiple mononeuropathies in seven patients (patients 1, 2, 3, 5, 6, 8, and 10), with brachial plexopathies in two patients (patients 4 and 7), and with mononeuropathies in three patients (patients 11, 12, and 13). Thirty-eight motor NCSs were performed in the affected nerves showing abnormal uptake on FDG-PET/CT scans (Supplementary Table 1 in the online-only Data Supplement). The results of the motor NCSs are summarized in Table 2. Ten (26%) motor NCSs revealed motor conduction abnormalities that met the criteria for a partial conduction block, and these patients were therefore allocated to the conduction-block group. The other 28 (74%) motor NCSs were assigned to the simple-axon-degradation group. The median time interval between symptom onset and NCSs was significantly shorter in the conduction-block group (median=29 days, IQR=10–68 days) than in the simple-axon-degradation group (median=48 days, IQR=29–107 days, p=0.032) (Fig. 1A). However, no significant differences in the motor NCVs, terminal latencies, or distal CMAP amplitudes were identified between the conduction-block and simple-axon-degradation groups (Supplementary Table 2 in the online-only Data Supplement). The conduction-block group showed excessive temporal dispersion in five (50%) of the ten tests (Fig. 1B and C). Inching studies demonstrated partial conduction blocks without delayed latencies at nonentrapment sites in patients 2 and 12 (Supplementary Fig. 3 in the online-only Data Supplement).
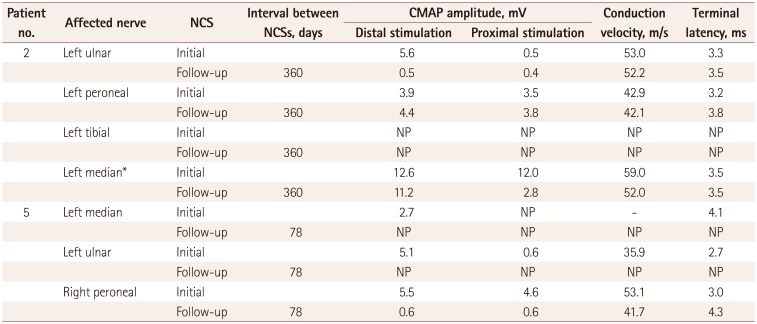
Partial conduction blocks were found in 7 (58%) of 12 patients with neurolymphomatosis. Partial conduction blocks were present at the site of increasing FDG uptake on FDG-PET/CT scans in six of these patients. The exception was patient 12, who showed a partial conduction block between the wrist and elbow segment, but focal FDG uptake was present in the upper arm on FDG-PET/CT scans (Supplementary Fig. 1B in the online-only Data Supplement). However, the patients with and without partial conduction blocks did not differ with regard to other clinical characteristics such as sex, onset age, underlying malignancy, affected neural structures, pain, and survival time. Two patients (patients 2 and 5) were initially misdiagnosed as multifocal acquired demyelinating sensory and motor neuropathy due to the presence of partial conduction blocks. Follow-up NCSs demonstrated that the initial partial conduction blocks had converted to axonal degeneration (Table 3, Fig. 2). Patient 2 received systemic chemotherapy during the interval from the first to the follow-up NCS, whereas patient 5 received steroid and immunoglobulin therapies instead of chemotherapy because he was diagnosed as neurolymphomatosis after the follow-up NCS.
The present study characterized the clinical manifestations, utility of various diagnostic modalities, and NCS findings in 13 patients with neurolymphomatosis. Nerve pathology and CSF cytology demonstrated malignant cells in 2 and 3 of the 13 patients, respectively. Nerve biopsy is not routinely performed in all patients with neurolymphomatosis because the affected lesions are frequently located in the deep-seated plexus, root, or cranial nerves, and because a nerve biopsy usually results in permanent motor weakness and sensory deficits. Additionally, the sensitivity of CSF cytology is very low, at 20–40%.115 Therefore, the other eight patients had their neurolymphomatosis pathologically confirmed at extraneural sites; additionally, MRI and FDG-PET/CT scans were used to identify the peripheral nerve infiltration that is associated with hematological malignancy.
The clinical presentations of the present patients with neurolymphomatosis were similar to those reported previously. 11215 The age at the onset of neurolymphomatosis was >50 years in 69% of the patients. All patients except patient 11 had painful and asymmetrical peripheral neuropathy involving damage to multiple nerves. Neurolymphomatosis was the first manifestation of hematological malignancy in one-third of the patients. About half of the patients had brain or cranial nerve involvement. The median time interval between symptom onset and diagnosis was about 2 months, and it was 8 months in patient 1. The median survival time was 7 months after diagnosis, and 12 patients died. Among the diagnostic modalities used, FDG-PET/CT and limb/plexus MRI scans performed well by demonstrating abnormalities in 100% and 83% of the patients, respectively. On the other hand, the sensitivity of CSF cytology was very low (38%), which is compatible with the findings of previous studies.389121617 A nerve biopsy did not pathologically confirm lymphoma infiltration in patient 8; this biopsy involved the sural nerve, which is a distal sensory branch of the sciatic nerve, and FDG-PET/CT scans of this patient revealed abnormal uptake at the proximal part of the sciatic nerve.
Partial conduction blocks were found in 58% of the patients in this study. This characteristic NCS finding has been occasionally reported in neurolymphomatosis.111218 A partial conduction block generally indicates that saltatory conduction has stopped even though the axon remains relatively intact. Therefore, previous authors have suggested that partial conduction block is linked to the infiltration pattern of hematological malignancy or humoral factors produced by lymphoma.12 Indeed, hematological malignancy occasionally infiltrates around the nerves–and not within the nerves–in several nerve pathologies.121819 However, the precise mechanism underlying a partial conduction block remains unclear.
Several aspects of our electrophysiological results suggest that the mechanism of partial conduction in neurolymphomatosis is axonal degeneration. First, none of the NCS findings except a partial conduction block differed significantly between the conduction-block and simple-axon-degradation groups. Second, partial conduction blocks were identified by NCSs performed within 3 months after symptom onset and had converted into simple-axon-degeneration patterns by the time of the follow-up NCSs. Third, the shape and pattern of partial conduction blocks in our patients with neurolymphomatosis differed from those identified in patients with demyelinating neuropathies. Partial conduction blocks without delayed latencies were found at nonentrapment sites in our neurolymphomatosis patients, while temporal dispersion was identified in only half of the patients. Finally, the nerve pathologies were compatible with axonal degeneration in both our own previous studies and those of others.121820 However, the partial conduction blocks lasted for longer in neurolymphomatosis than in other axonal neuropathies. Partial conduction blocks have been reported as a transient NCS finding in the early phase of axonal neuropathies.212223
The pathophysiology of a partial conduction block in acute motor axonal neuropathy is assumed to be associated with complement deposits at the nodes of Ranvier.24 This characteristic finding in vasculitic neuropathy is assumed to be related to metabolic block, paranodal demyelination, segmental demyelination, and Wallerian degeneration, which reflect different degrees of ischemic damage.2223 Partial conduction blocks are usually identified less than a week after symptom onset,212223 while the partial conduction blocks in the present study lasted for 3 months after symptom onset in neurolymphomatosis. We therefore assume that partial conduction in neurolymphomatosis might be caused by both axonal degeneration and segmental demyelination rather than by a single pathomechanism.
It is important to note that this was a small retrospective study and as such does not provide sufficient evidence regarding the pathomechanism of a partial conduction block in patients with neurolymphomatosis. Further prospective studies are therefore needed to confirm our results in larger cohorts and to identify the pathogenic mechanisms of a partial conduction block in neurolymphomatosis.
Collectively, the results of the present study demonstrate that partial conduction blocks are a temporary NCS finding in about half of all patients with neurolymphomatosis. Partial conduction blocks frequently result in delayed diagnoses or misdiagnoses of neurolymphomatosis, which in turn leads to the spread of the malignancy and a poor prognosis. Clinicians therefore need to be aware that neurolymphomatosis can be associated with partial conduction blocks, especially in patients with painful asymmetric neuropathies or multiple mononeuropathies. In conclusion, the present study improves our understanding of the clinical and electrophysiological findings associated with neurolymphomatosis, and is the first study to identify a partial conduction block as an early nerve conduction abnormality in patients with neurolymphomatosis.
Acknowledgements
This research was supported by the Basic Science Research Program though the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2016R1D1A1B03932449).
References
1. Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010; 115:5005–5011. PMID: 20368468.

2. Jellinger K, Radiaszkiewicz T. Involvement of the central nervous system in malignant lymphomas. Virchows Arch A Pathol Anat Histol. 1976; 370:345–362. PMID: 826017.

3. Matsue K, Hayama BY, Iwama K, Koyama T, Fujiwara H, Yamakura M, et al. High frequency of neurolymphomatosis as a relapse disease of intravascular large B-cell lymphoma. Cancer. 2011; 117:4512–4521. PMID: 21448935.

4. Pillay PK, Hardy RW Jr, Wilbourn AJ, Tubbs RR, Lederman RJ. Solitary primary lymphoma of the sciatic nerve: case report. Neurosurgery. 1988; 23:370–371. PMID: 3226515.

5. Hughes RA, Britton T, Richards M. Effects of lymphoma on the peripheral nervous system. J R Soc Med. 1994; 87:526–530. PMID: 7932460.
6. Kelly JJ, Karcher DS. Lymphoma and peripheral neuropathy: a clinical review. Muscle Nerve. 2005; 31:301–313. PMID: 15543550.

7. Chang GY. Evolution of neurolymphomatosis to lymphomatosis cerebri. J Clin Neurol. 2017; 13:203–204. PMID: 28176503.

8. von Falck C, Rodt T, Joerdens S, Waldeck S, Kiesel H, Knapp WH, et al. F-18 2-fluoro-2-deoxy-glucose positron emission tomography/computed tomography for the detection of radicular and peripheral neurolymphomatosis: correlation with magnetic resonance imaging and ultrasound. Clin Nucl Med. 2009; 34:493–495. PMID: 19617723.
9. Gan HK, Azad A, Cher L, Mitchell PL. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro Oncol. 2010; 12:212–215. PMID: 20150388.

10. Kinoshita H, Yamakado H, Kitano T, Kitamura A, Yamashita H, Miyamoto M, et al. Diagnostic utility of FDG-PET in neurolymphomatosis: report of five cases. J Neurol. 2016; 263:1719–1726. PMID: 27286845.

11. Sekiya H, Sekiguchi K, Noda Y, Kowa H, Kanda F, Toda T. Conduction block in neurolymphomatosis. Muscle Nerve. 2015; 52:S117.
12. Tomita M, Koike H, Kawagashira Y, Iijima M, Adachi H, Taguchi J, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013; 136(Pt 8):2563–2578. PMID: 23884813.

13. Oh SJ. Clinical Electromyography: Nerve Conduction Studies. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins;2003.
14. American Association of Electrodiagnostic Medicine. Olney RK. Guidelines in electrodiagnostic medicine. Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve Suppl. 1999; 8:S225–S229. PMID: 16921636.
15. Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro Oncol. 2003; 5:104–115. PMID: 12672282.

16. Shima K, Ishida C, Okino S, Kotani T, Higashi K, Yamada M. A linear lesion along the brachial plexus on FDG-PET in neurolymphomatosis. Intern Med. 2008; 47:1159–1160. PMID: 18552480.

17. Oztürk E, Arpaci F, Kocaoğlu M, Arslan N, Bulakbaşi N, Ozgüven M. Detection of widespread neurolymphomatosis with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2006; 33:975–976. PMID: 16703365.

18. Groth CL, Nevel KS, Gwathmey KG, Bafakih F, Jones DE. Splenic marginal zone lymphoma: an indolent malignancy leading to the development of neurolymphomatosis. Muscle Nerve. 2017; 55:440–444. PMID: 27625159.

19. Lagarde S, Tabouret E, Matta M, Franques J, Attarian S, Pouget J, et al. Primary neurolymphomatosis diagnosis and treatment: a retrospective study. J Neurol Sci. 2014; 342(1-2):178–181. PMID: 24831985.

20. Shree R, Goyal MK, Modi M, Gaspar BL, Radotra BD, Ahuja CK, et al. The diagnostic dilemma of neurolymphomatosis. J Clin Neurol. 2016; 12:274–281. PMID: 27449910.

21. Kokubun N, Nishibayashi M, Uncini A, Odaka M, Hirata K, Yuki N. Conduction block in acute motor axonal neuropathy. Brain. 2010; 133:2897–2908. PMID: 20855419.

22. McCluskey L, Feinberg D, Cantor C, Bird S. “Pseudo-conduction block” in vasculitic neuropathy. Muscle Nerve. 1999; 22:1361–1366. PMID: 10487901.

23. Ropert A, Metral S. Conduction block in neuropathies with necrotizing vasculitis. Muscle Nerve. 1990; 13:102–105. PMID: 1969113.

24. Griffin JW, Li CY, Macko C, Ho TW, Hsieh ST, Xue P, et al. Early nodal changes in the acute motor axonal neuropathy pattern of the Guillain-Barré syndrome. J Neurocytol. 1996; 25:33–51. PMID: 8852937.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.1.73.
Supplementary Fig. 1
FDG positron-emission tomography/computed tomography images of patients with neurolymphomatosis. A: Patient 5 demonstrated increased linear FDG uptake in the left brachial plexus and right lumbosacral plexus (black arrows). B: Patient 12 showed abnormal focal FDG uptake in the left brachial plexus and ulnar nerve (white arrowheads). FDG: 18F-2-fluoro-2-deoxy-D-glucose.
Supplementary Fig. 2
Nerve biopsy samples were obtained from left superficial radial nerve in patient 5 (A and B) and left median nerves in patient 10 (C and D). A: Staining with hematoxylin and eosin (H&E) revealed atypical lymphocytic infiltrations mainly in the perineurium. B: Immunohistochemistry for CD20 (a B-cell marker) was positive in lymphocytes. C: H&E stain showed that large lymphoid cells exhibited marked variations in size and shape. D: Immunohistochemistry for CD20 was positive in atypical lymphocytes. Original magnification: A and B, ×200; and C and D, ×400.
Supplementary Fig. 3
Results of inching studies of the ulnar nerves. A: An inching study of the left ulnar nerve showed a 57% decrease in amplitude without delayed latencies between 6 cm and 8 cm proximal to the distal wrist crease in patient 2. B: An inching study of the left ulnar nerve showed a 46% decrease in amplitude without delayed latencies between 5 cm and 6 cm proximal to the distal wrist crease in patient 12.
Supplementary Table 2.
Comparison of NCS results in conduction-block and axon-degradation groups
Fig. 1
A partial conduction block in neurolymphomatosis. A: The median time interval between symptom onset and the NCS was significantly shorter in the conduction-block group than in the simple-axon-degradation group. B and C: A median motor NCS of patient 2 demonstrated a partial conduction block with excessive temporal dispersion (B), while an ulnar motor NCS of patient 6 revealed a partial conduction block without dispersion (C). NCS: nerve conduction study.
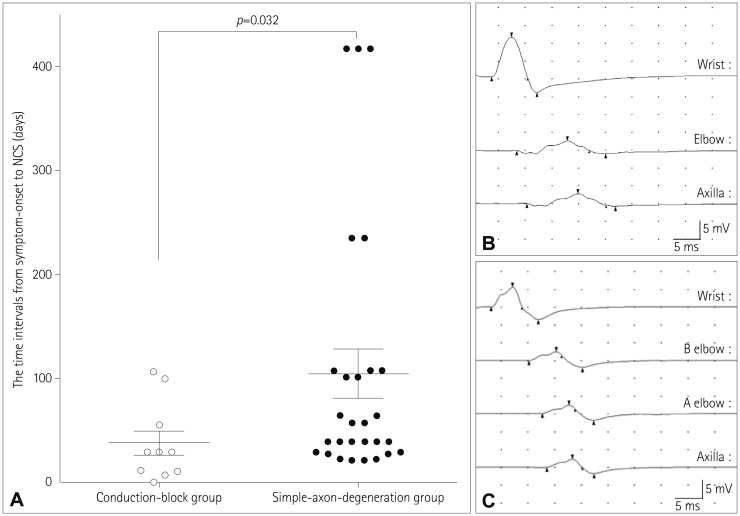
Fig. 2
Findings of follow-up NCSs of the affected nerves with a partial conduction block. In patient 2, the initial motor NCS of the left ulnar nerve demonstrated a partial conduction block (A), but the follow-up NCS performed 360 days later produced results compatible with axon degeneration (B). In patient 5, conduction blocks were found during the initial motor NCS of the left median (C) and ulnar (E) nerves, while the NCS performed 78 days later did not show potentials (D and F). NCS: nerve conduction study.
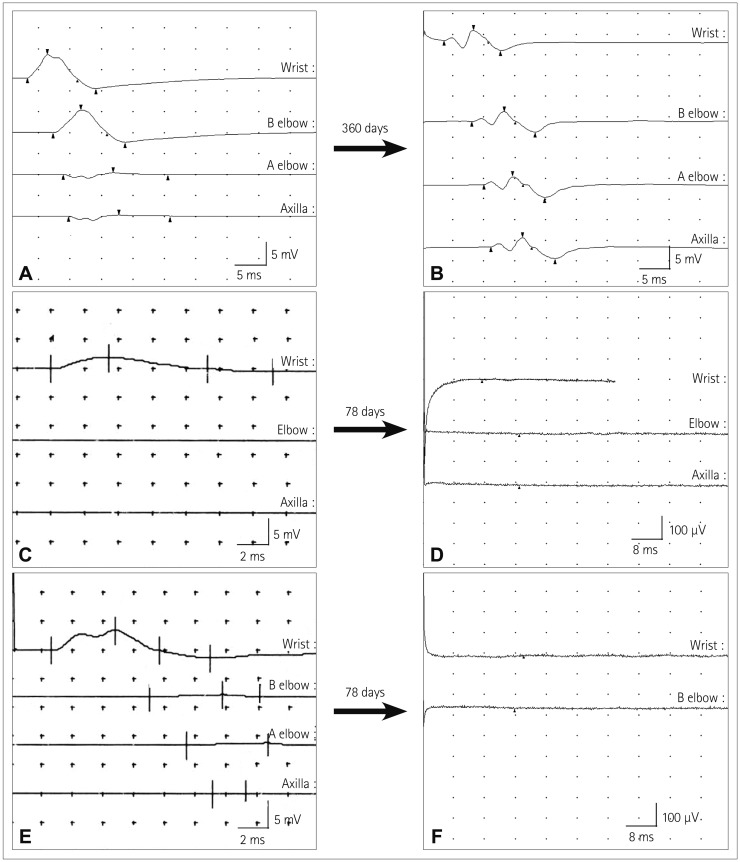
Table 1
Clinical features of and diagnostic modalities used in 13 patients with NL
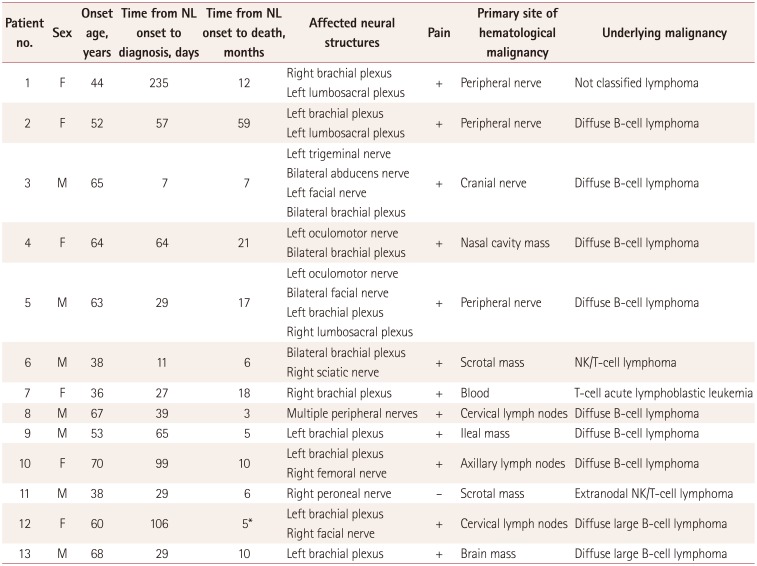
Table 2
Results of motor nerve conduction studies
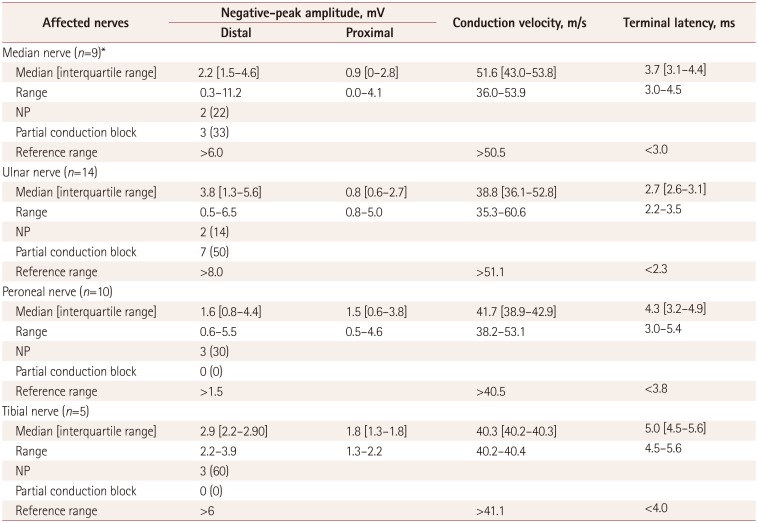
Table 3
Results of follow-up NCSs




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download