INTRODUCTION
The Parks-Bielschowsky three-step test is the mainstay for identifying isolated palsied cyclovertical muscles, especially in patients with superior oblique palsy (SOP).
12 However, this test has limitations due to mimicking clinical conditions,
3456 and so the test results need to be interpreted cautiously and should be used in combination with supplementary findings when making definitive diagnoses. Moreover, the validity of test has not been generally investigated. Several previous studies restrictively evaluated the sensitivities of the three-step test in SOP in conjunction with magnetic resonance imaging (MRI) profiles.
78 Demer et al.
7 documented that only half of patients exhibiting a complete three-step test had a hypoplastic superior oblique muscle (SO), suggesting that the specificity of the test is low in SOP patients. Another study found that 30% patients with SO atrophy failed to fulfill the entire three-step test, indicating a sensitivity of 70%.
8 Although a smaller SO represents confirmatory evidence of SOP, this corresponds to only two-thirds of patients with congenital SOP
910 and up to 75% of those with idiopathic SOP.
11 Since the absence of the trochlear nerve represents definite evidence of SO palsy, the sensitivity of the three-step test needs to be clarified according to the absence or presence of the trochlear nerve.
To our knowledge, no previous study has investigated the relationship between the existence of the trochlear nerve and the diagnostic sensitivity of the three-step test. The first aim of the present study was to establish the sensitivity of this test according to the existence of the trochlear nerve. The second aim was to determine the characteristics of the patients who exhibited a completely positive three-step test.
METHODS
Approval to conduct this study was obtained from the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1511-322-102). All aspects of the research protocol and clinical investigation were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was not required, since patient records and information were anonymized and de-identified prior to the analysis.
Subjects
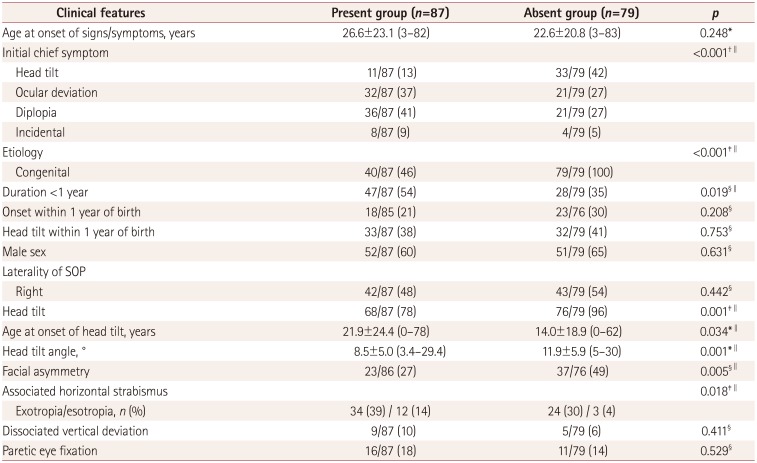
This study conducted a retrospective review of the medical records of 166 consecutive patients who were diagnosed with unilateral SOP and had undergone high-resolution thin-section MRI at Seoul National University Bundang Hospital between January 2012 and August 2015. The diagnosis of unilateral SOP was made by applying the following three absolute criteria: 1) unilateral under-depression in addition to the presence or absence of overelevation in adduction, 2) hyperdeviation in the primary position on the alternate prism cover test, and 3) no evidence of other ocular motility disorders causing vertical deviation, including contracture of the vertical rectus muscle except for secondary contracture of the superior rectus muscle (SR) associated with SOP, paresis of multiple vertical muscles, previous muscle surgery, skew deviation, myasthenia gravis, vertical-deviation-related horizontal strabismus, and structural abnormalities such as muscle pulley heterotopy or craniosynostosis. The etiology was classified as “congenital” based on photographic or reliable historical documentation of a long-standing strabismus or an ocular torticollis since infancy or early childhood, large fusional amplitudes of vertical deviation, and the absence of torsional diplopia. The etiology was classified as “acquired” when there was no previous occurrence, normal vertical fusional amplitudes, presence of torsional diplopia, and suspicious history of a preceding event typically related to the onset of symptoms.
Clinical measurements
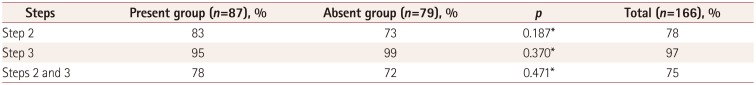
Clinical characteristics were collected and all study patients underwent complete ophthalmologic examinations. The examinations included alternate prism cover measurements in the six diagnostic positions of gaze and the Bielschowsky head-tilt test. To detect the smallest differences so as to maximize the sensitivity, each step was considered “positive” in the presence of (Step 1) hyperdeviation in the primary position, (Step 2) hyperdeviation greater by at least 1 prism diopter (PD) in contralateral gaze than ipsilateral gaze, and (Step 3) positive Bielschowsky head-tilt test with an increment of the hyperdeviation by at least 1 PD on ipsilateral head tilt compared to contralateral head-tilt test. The ocular motility of the oblique muscles was graded based on a subjective scale scored from 0 to 4 of underaction (−) or overaction (+).
The three-step test was performed by two independent ophthalmologists before performing MRI scans or any other ancillary diagnostic tests. SR overaction or contracture was defined by the following previously published clinical criteria:
1213 a vertical deviation of 15 PD or more in the primary position, hypertropia that is the same as or larger with ipsilateral head tilt than with the eyes looking straight ahead, more than 5 PD hypertropia of the affected eye in gaze to the side of the SO palsy, hypertropia in all upgazes, and overaction of the contralateral SO of +1 or more. Laterality of the paretic eye, fixation dominance, dissociated vertical deviation and associated horizontal strabismus were also noted. Subjective torsion in the primary gaze was assessed with double Maddox rods. Objective ocular torsion was evaluated by funduscopy or in fundus photographs obtained using fundus cameras (KOWA VX-10, Kowa Company, Tokyo, Japan; or TRC-50IA, Topcon, Tokyo, Japan) with internal fixation.
14
MRI: trochlear nerve and superior oblique muscle area
MRI was performed using a 3-T system (Intera Achieva, Philips Healthcare, Best, the Netherlands) with an eight-channel sensitivity encoding head coil in a protocol that was identical to one we described previously.
91516 The optic nerve-globe junction was defined as the standard point, and SO areas were measured in the T2-weighted coronal orbital MR image at the standard point using Picture Archiving and Communication System (PACS) software (INFINITT, Seoul, Korea).
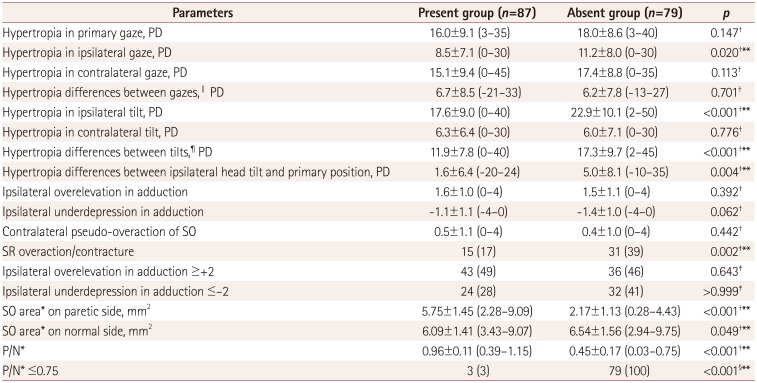
10 Recent research data support that a ratio of the SO areas on the paretic and normal sides (P/N) at the optic nerve-globe junction of ≤0.75 is highly predictive of trochlear nerve absence in congenital SOP.
10 Thus, a P/N of ≤0.75 was used as the cutoff value indicating clinically significant SO atrophy in the present study.
Statistical analyses
Statistical analyses were performed with SPSS software (version 20, IBM Corp., Armonk, NY, USA).
We compared groups using the independent t test for continuous variables, and Pearson's chi-square test, Fisher's exact test, or the likelihood ratio test for trend for categorical data. Univariate and multivariate analyses using logistic regression were applied to identify factors associated with the results of the three-step test. A probability value of p<0.05 was considered to be indicative of statistical significance.
DISCUSSION
This study found that patients with clinically diagnosed unilateral SOP showed a sensitivity of 75% in the three-step test regardless of the presence of the trochlear nerve or significant SO atrophy on MRI. To the best of our knowledge, this is the first series of SOP patients with complete documentation for the three-step test according to visualization of the trochlear nerve.
The diagnostic excellence of the three-step test depends on the assumption that palsy is present in only one cyclovertical muscle.
3 The validity of tests for SOP is reduced by some masquerading conditions that induce false-positive results.
3456 The paucity of comprehensive assessments of the validity of the test may be due to the lack of available data related to the fundamental etiology of SOP–the existence of the trochlear nerve. We therefore assume that the diagnostic value of the test could be evaluated accurately based on the pathologic etiology of trochlear nerve absence rather than SO morphology on MRI or by experimental test results as done in previous researches.
78 Thus, in contrast to the study of Manchandia and Demer,
8 the present study included patients lacking definite SO atrophy but with a strong clinical implication of unilateral SOP. Moreover, we also included young children (14 patients, age range=3–4 years) who were able to perform the alternate prism cover test for cardinal gazes, which are critical for a definitive diagnosis.
We previously demonstrated that the two main etiologies according to the existence of the trochlear nerve in congenital SOP showed distinctively different clinical features.
9 The absence of the trochlear nerve represents clear-cut evidence of congenital SOP represented by SO atrophy and variable degrees of hypoplasia of the SO on the paretic side.
910151718 Acquired SOP may also be associated with SO atrophy as in the absent group of congenital SOP; however, the trochlear nerve would be normal or hypoplastic, but not absent.
19 Regarding SO atrophy, a previous study found no significant morphologic difference of the SO on MRI between acquired and congenital SOP.
19 However, a recent experimental study found that SO atrophy in acquired SOP was not profound because mainly the global layer fibers were atrophied to 20% of their normal size, and the remaining SO fibers in the orbital layer maintained their normal size.
20 The present study also found that the size of the SO on the paretic side was reduced more in the congenital group (3.42±2.19 mm
2, mean±standard deviation; range=0.28–8.58 mm
2) than in the acquired group (5.64±1.31 mm
2, range=2.28–9.09 mm
2) (
p<0.001). A subgroup analysis found a negative correlation between the SO size and the difference in hypertropia between ipsilateral head tilt and the primary position (
r=−0.222,
p=0.004). Previous studies have estimated SO function using two main methods: MRI morphometry and ocular motility parameters. The SO cross-sectional area on MRI is positively correlated with contractility,
21 ocular torsion in response to head tilting, or static ocular counterrolling,
22 but not with the Bielschowsky head tilt phenomenon.
20 Hamasaki et al.
22 reported that the differences in hypertropia between ipsilateral head tilt and the upright position reflected SO functioning rather than the absolute value of hypertropia in ipsilateral head tilt. These results suggest that fundamental etiologic differences result in a heterogeneous and unclear cause-and-effect relationship, and furthermore contribute to differences in the clinical characteristics of congenital SOP.
In the present investigation, the absent group showed consistent findings including larger hypertropia during ipsilateral gaze and ipsilateral tilt, as described previously.
9 These findings strongly support the incidence of ipsilateral SR overaction or contracture being higher in the absent group than in the present group, resulting in failure of step 2. In addition, the amount of ipsilateral overelevation in adduction and the proportion of ipsilateral overelevation in adduction ≥+2 were similar in both the absent and present groups. Kono and Demer
21 documented that the ipsilateral antagonist inferior oblique muscle (IO) in unilateral SOP presented neither hypertrophic nor supernormal contractility. Their report is consistent with our finding of no significant increment of ipsilateral overelevation on adduction, regardless of the absence of the trochlear nerve or size of the SO. We may therefore expect that SOP–independent of the presence of the trochlear nerve or degree of SO atrophy–does not necessarily accompany the compensatory overaction of the IO, which is represented by the increment of hypertropia on contralateral gaze that is the most frequent step (step 2) in which failure occurs.
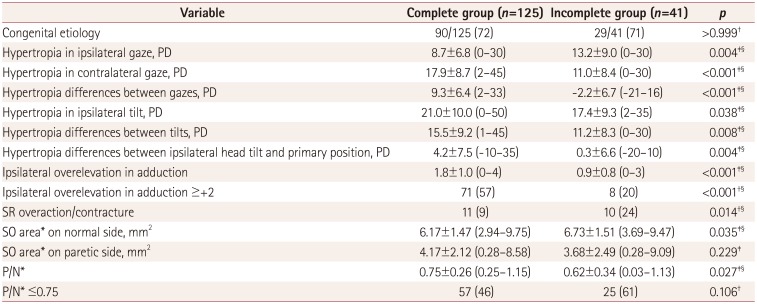
Hypertropia differences between both gazes as well as both tilt gazes and the amount of ipsilateral overelevation in adduction were remarkably larger in the complete group than in the incomplete group. Some of these parameters are regarded as relevant factors for a positive result for step 2. The size of the SO on the paretic side was similar in the two groups, reflecting that SO contractility might not directly affect positive results in the three-step test. Although SR overaction or contracture was less frequent in the complete group, there was larger hypertropia during ipsilateral tilt in that group. Heterogeneous biomechanical alterations might also influence hyperdeviation patterns in both gazes and head tilt.
102123 Together these observations suggest that the three-step test can elicit a complex response that depends not only on SO weakness itself but also on serial changes in the ipsilateral antagonist or other muscles due to additional mechanical and innervation changes. The results of the three-step test might therefore not solely reflect the original tone of the SO.
Some limitations of this analysis need to be considered. First, the diagnosis of SOP partially was based on clinical criteria. In patients with an ipsilateral SO of normal size and a normal trochlear nerve who were not diagnostically supported by MRI, we attempted to obtain clinical evidence of SO underaction while excluding the other possible causes mentioned. According to Bielschowsky, if a single muscle becomes paretic, deviation in the primary position is always present, being latent or manifest according to the intensity of the paresis and the function of the paretic muscle.
1 It is known that paresis of the SO induces hyperdeviation of the affected eye. However, some patients with SO atrophy may not exhibit hypertropia in the primary position due to their large fusional capacity. A recent study found that 8% of patients with SO atrophy on MRI did not show hypertropia in the primary gaze.
8 Considering that the present study excluded such patients who exhibited a negative result for step 1, the true sensitivity of the three-step test would be lower if these patients were included, which is consistent with our qualitative conclusions. Nonetheless, we previously identified that all patients without a trochlear nerve exhibited hypertropia in the primary position when they were examined after applying sufficient occlusion to the involved eye.
24 We occluded the involved eye for 30–60 minutes in order to induce a long period of dissociation before performing the alternate prism cover test. This method allowed us to detect even the smallest amount of latent deviation. This is the most distinguishing feature from the 2014 study of Manchandia and Demer,
8 because we regarded the first step as an essential component for diagnosis if tested after sufficient occlusion to reveal all possible latent hyperdeviation. Based on this background, we tried to evaluate not only the sensitivity of the three-step test but also other relevant factors related to the accuracy of the test, especially the presence or absence of the trochlear nerve.
Second, to optimize the sensitivity we adopted the definition by Manchandia and Demer
8 of a “positive step” being a difference in hypertropia between ipsilateral and contralateral gaze or both head tilt of at least 1 PD. Regarding the variability of the alternate prism cover test,
25 these criteria may overestimate the sensitivity and underestimate the specificity of the test by producing high false-positive rates in cases simulating unilateral SOP. Further studies that include diverse etiologies of hypertropia should be carried out to determine the specificity of the three-step test in diagnosing unilateral SOP.
37
Third, including patients with secondary SR overaction/contracture associated with SOP in this study might have affected the test sensitivity. We selectively included these patients when obvious evidence of unilateral SOP such as definite MRI findings of SO atrophy or trochlear nerve absence, ductions, versions and/or fundus excyclotorsion were confirmed. In addition, a negative result for step 2 was documented as the most relevant finding of SR overaction/contracture syndrome in unilateral SOP.
13 The present study found that the proportion of patients with SR overaction or contracture was higher in the incomplete group. Although the absent group had a significantly larger proportion of patients with SR overaction/contracture than the present group (39% vs. 17%,
p=0.002), the rate of positive results for step 2 did not differ significantly between the absent and present groups (73% vs. 83%,
p=0.187)
Finally, a large proportion of the children who were among the 321 patients clinically diagnosed as unilateral SOP were too young for the angle of deviation to be examined in all six cardinal positions of gaze due to poor cooperation, and the trochlear nerve was absent in most of them. Since the angles of deviation in the six diagnostic positions of gaze are critical elements of the main outcome measure in our study, it was inevitable that very young children would be excluded. This situation might have introduced bias into the results of our study. However, since young children are not able to perform the three-step test, the clinical utility of this test is not applicable to these patients, and so the qualitative conclusions from our results might not be influenced by the exclusion of young uncooperative patients.
In conclusion, the three-step test had a sensitivity of 75% in diagnosing unilateral SOP, regardless of the presence of the trochlear nerve. The results of the three-step test do not seem to differ according to the presence or absence of the trochlear nerve. The combination of steps 1 and 3 was more sensitive than all three steps in diagnosing unilateral SOP. SR overaction/contracture was the main cause of exceptions in the threestep test results in unilateral SOP.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download