Abstract
Background and Purpose
Benign childhood epilepsy with centrotemporal spikes (BECTS) does not always have a benign cognitive outcome. We investigated the relationship between cognitive performance and altered functional connectivity (FC) in the resting-state brain networks of BECTS patients.
Methods
We studied 42 subjects, comprising 19 BECTS patients and 23 healthy controls. Cognitive performance was assessed using the Korean version of the Wechsler Intelligence Scale for Children-III, in addition to verbal and visuospatial memory tests and executive function tests. Resting-state functional magnetic resonance imaging was acquired in addition to high-resolution structural data. We selected Rolandic and language-related areas as regions of interest (ROIs) and analyzed the seed-based FC to voxels throughout the brain. We evaluated the correlations between the neuropsychological test scores and seed-based FC values using the same ROIs.
Results
The verbal intelligence quotient (VIQ) and full-scale intelligence quotient (FSIQ) were lower in BECTS patients than in healthy controls (p<0.001). The prevalence of subjects with a higher performance IQ than VIQ was significantly higher in BECTS patients than in healthy controls (73.7% vs. 26.1%, respectively; p=0.002). Both the Rolandic and language-related ROIs exhibited more enhanced FC to voxels in the left inferior temporal gyrus in BECTS patients than in healthy controls. A particularly interestingly finding was that the enhanced FC was correlated with lower cognitive performance as measured by the VIQ and the FSIQ in both patients and control subjects.
Cognitive impairment is one of the most common comorbid disorders in childhood epilepsies, and its negative effects on the immature brain can be more deleterious than seizures.123 The high prevalence of cognitive comorbidities even in newly diagnosed drug-naïve patients suggests the presence of intrinsic abnormalities attributable to genetic factors or inherited traits of the epileptogenic brain.4567 However, certain epilepsy syndromes are associated with gradual cognitive or behavioral decline as the seizures and epileptiform discharges (EDs) are prolonged. Furthermore, the causal relationships between epilepsy-related factors [e.g., etiology, onset age, seizure type and frequency, disease duration, and antiepileptic drugs (AEDs)] and cognition have not yet been clearly delineated.
Recent progress in neuroimaging techniques has provided some evidence of underlying neurobiological mechanisms, especially related to microstructural or functional alterations of the brain. Our previous studies also showed that although childhood epilepsy patients appeared to have a normal brain structure on visual inspection of the images obtained using magnetic resonance imaging (MRI), they exhibited microstructural abnormalities correlated to poor cognition when assessed using diffusion tensor imaging (DTI) or voxel-based morphometry (VBM) methods.58 Studies of altered functional connectivity (FC) in pediatric epilepsy patients are becoming more common, mainly concerning the resting-state brain network by measuring the degree of synchronization in slow blood-oxygen-level-dependent (BOLD) signal fluctuations.91011 Resting-state functional magnetic resonance imaging (rs-fMRI) is particularly useful in young children with or without cognitive problem since no specific task needs to be performed.
This study focused on patients with benign childhood epilepsy with centrotemporal spikes (BECTS), which is the most common childhood epilepsy syndrome and has been considered a relatively benign disorder due to spontaneous seizure remission occurring in almost 90% of patients.1213 However, the evidence accumulated over the last decade has indicated that BECTS might not be as benign as originally thought, with several studies demonstrating associated deficits in intelligence, attention, and executive function.14 Furthermore, even the current diagnostic criteria of BECTS do not include structural lesions, while recent functional magnetic resonance imaging (fMRI) studies have revealed altered FC between various regions in large-scale brain networks.151617181920
The first goal of the present study was to identify differences in the resting-state FC patterns between BECTS patients and healthy controls. Moreover, since we hypothesized that the effects of FC alterations between specific brain areas would be correlated with cognitive impairments in BECTS patients, the intellectual functioning in various cognitive domains was tested and its relationships with FC changes were also assessed. Since several studies have demonstrated that intellectual problems in BECTS patients are mainly linked to language function, our regions of interest (ROIs) included not only the epileptogenic Rolandic cortex but also language-related areas.1617212223
We recruited children and adolescents who had visited the epilepsy clinic of Ewha Womans University Mokdong Hospital. The diagnosis of epilepsy and determination of epileptic syndrome were made based on the clinical history, electroencephalography (EEG) features, and neuroradiological findings by expert neurologists (H.J.K., J.H.L., and H.W.L.).2425 Structural lesions identified by the visual inspection of images obtained using MRI, neuropsychiatric disorders that could influence cognitive function (e.g., psychotic disorder, autism spectrum disorder, or mental retardation), and other chronic illness were considered as exclusion criteria. However, patients with attention deficit hyperactivity disorder were not excluded since these patients were able to follow the standard school curriculum.
Twenty-four patients suffering from BECTS were initially included in this study. They were elementary-, middle-, or high-school students who were able to understand and answer neuropsychological test questionnaires. However, four subjects with an intelligence quotient (IQ) of less than 70 were subsequently excluded. The epileptogenic hemisphere of each patient was determined based on EEG abnormalities detected at the time of diagnosis. Routine EEG data were re-examined at the time of the rs-fMRI study (within 3 months) to confirm the epileptogenic hemisphere, in addition to subcategorizing the patients based on the frequency of EDs.
We also recruited 25 healthy participants as a control group with age, sex, and education distributions that were similar to those in the patient group. Individuals with any past or current medical issues including epilepsy and neuropsychiatric disorders, a history of febrile convulsion, or a family history of epilepsy in first-degree relatives were excluded from the control group.
This study was approved by the Human Investigation Committee of the Ewha Womans University Medical Center (IRB No. 2010-001-233-2). Written informed consent was obtained from all participants and/or their parents.
All of the study participants underwent comprehensive neuropsychological testing. General intelligence was evaluated using the Korean version of the Wechsler Intelligence Scale for Children-III (K-WISC-III). The parameters assessed were verbal intelligence quotient (VIQ), performance intelligence quotient (PIQ), and full-scale intelligence quotient (FSIQ), and four factorial subscales consisting of a verbal comprehension index (VCI), perceptual organization index (POI), freedom from distractibility index (FDI), and processing speed.26 The Rey Auditory Verbal Learning Test (AVLT) and Rey-Osterrieth Complex Figure Test (RCFT) were used to assess verbal and visuospatial memory functioning, respectively.2728 Executive functioning was assessed by the Trail-Making Test Parts A and B and the Stroop Color-Word association tests, which measure attention and the speed of cognitive processing.2930 All raw scores from the above tests were converted into age-adjusted scores.
MRI data were acquired with a 3.0-tesla scanner (Achieva TX series, Philips, Best, the Netherlands) equipped with T2*-weighted gradient echo planar imaging capabilities. Functional images of 150 volumes with BOLD contrast in the axial plane were collected using the following parameters: repetition time=2,000 ms, echo time=32 ms, in-plane resolution=1.67×1.67 mm, matrix=144×144, and slice thickness=5.00 mm (32 slices). The subjects were instructed to close their eyes and remain as motionless as possible during scanning.
High-resolution structural images were also obtained during the same imaging methodology using the following parameters: in-plane resolution=0.50×0.50 mm, matrix=448×448, and slice thickness=1.00 mm (160 slices). Images were acquired in the coronal plane using a 3D sensitivity encoding sequence for the anatomical images, and in the axial plane using an echo planar imaging sequence for the functional images.
Preprocessing of rs-fMRI data was performed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/), and included the removal of head movement artifacts, spatial normalization to the same coordinate frame as the template brain conforming to the Montreal Neurological Institute (MNI) space, and spatial smoothing with a Gaussian kernel of 4 mm full-width-at-half-maximum. Transformation parameters for spatial normalization were derived from segmentation of the high-resolution structural image coregistered to the mean functional image. Additionally, DPARSF (http://rfmri.org/DPARSF) was used to remove linear trends due primarily to systematic increases or decreases in the signal, to regress out nuisance covariates, and to filter at 0.01–0.08 Hz. Six head movement parameters estimated during spatial realignment and cerebrospinal fluid and white matter signals were included as nuisance covariates.
During the preprocessing process, rs-fMRI data of one patient and two controls were excluded due to head movements that were larger than the voxel size. Thus, the preprocessed signals from 19 patients and 23 controls were finally used for the seed-based analyses performed in the next step.
We created seed masks of the Rolandic and language regions using MAsks for Region of INterest Analysis software (MARINA, version 0.6.1, B. Walter, Giessen, Germany). The Rolandic mask covered the bilateral inferior frontal gyri (pars triangularis and pars opercularis) and the Rolandic operculum and insula. The language area includes the bilateral inferior frontal gyri, middle and superior temporal gyri, and supramarginal gyri in addition to the left angular gyrus and left cerebellar crus I.31323334
These seed masks were subsequently transformed from the standard MNI space into the individual functional brain space of each subject. The BOLD time series of the voxels within each seed region were averaged to generate the reference time series for that seed. A correlation map was produced for each subject and each seed region by computing the correlation coefficient between the reference time series and the time series of all brain voxels. Correlation coefficients were converted into z values using Fisher's Z transform to improve the normality of the data.
Each individual Z value was entered into a random-effects one-sample t-test to identify brain regions showing significant connectivity to the Rolandic or language seed regions within each BECTS patient or control subject. Significant clusters were determined at the combination of the voxel-level height threshold of a p-value of 0.05 false discovery rate (FDR) corrected for multiple comparisons and the cluster-level extent threshold of a p-value of 0.05 FDR corrected for multiple comparisons. The Z values were also entered into a random-effects two-sample t-test to identify differences in FC between BECTS patients and controls. Voxels survived a p-value of 0.05 FDR corrected for multiple comparisons at the voxel-level were considered to show significant difference between the two groups. The anatomical localization of significant clusters was identified using MNI coordinates. To investigate whether the Rolandic or language FC was correlated with cognitive performance in our subjects, the value of each voxel from the fMRI seed-based FC maps was extracted and then correlated with cognitive test scores. Bonferroni correction for multiple comparisons was performed using a corrected p-value threshold of 0.0071 (=0.05/7) for the seven intellectual subdomains with significant group differences, which were included in the final correlation analyses.
Statistical analyses of the clinical characteristics and neuropsychological test scores were performed using SPSS (version 21.0, IBM Corp., Armonk, NY, USA). Chi-square and independent t-tests were used to compare categorical and continuous variables, respectively. A two-sided p value of less than 0.05 was considered to indicate statistical significance.
The clinical characteristics of the 19 BECTS patients and 23 healthy controls included in the final analyses are summarized in Table 1. There were no group differences in sex or age distribution at the time of study (p=0.382 and 0.122, respectively). The age of the BECTS patients at seizure onset was 7.37±2.71 years (mean±standard deviation, range=3–12 years), their clinical seizure duration was 30.89±40.83 months (range=0–135 months), and the duration of AEDs was 57.42±19.26 months (range=28–104 months) at the time of the rsfMRI study. The most commonly prescribed AEDs were oxcarbazepine (5/19, 26.3%) and valproic acid (5/19, 26.3%), with daily dosage ranges of 300–600 mg and 400–1,000 mg, respectively. Other AEDs included carbamazepine (2/19, 10.5%) and lamotrigine (2/19, 10.5%) at 400 mg and 100–200 mg daily, respectively. Three patients were not on AEDs, and the other two patients were on dual AED therapy because they were partly through AED switching (Oxcarbazepine 300 mg plus Valproic acid 450 mg, Lamotrigine 200 mg plus Valproic acid 250 mg daily, respectively).
The initial EEG study at the time of diagnosis revealed very frequent EDs on the left (8/19, 42.1%), right (4/19, 21.1%), or either hemisphere (7/19, 36.8%). The routine EEG study at the time of the rs-fMRI study revealed frequent (>1 per minute) or abundant (≥1 per 10 seconds) EDs in only 8 patients (42.1%), with the other 11 patients (57.9%) exhibiting no or only rare (≤1 per 10 minutes) EDs in EEG.
Table 2 compares the neuropsychological performance scores between the BECTS patients and health controls. General intelligence scores including VIQ, PIQ, and FSIQ evaluated using the K-WISC-III were significantly lower in the BECTS patients than in the healthy controls (p<0.001). The absolute verbal-performance intelligence quotient discrepancy (V-P) was 11.16±8.88 (range=1–30) for the BECTS patients and 10.48±7.99 (range=0–23) for the controls (p=0.795), regardless of the direction of the discrepancy. A discrepancy of 13 points or more, which is often considered to be statistically significant in the general population (31.7%),2635 occurred in 31.6% (6/19) of patients and 34.8% (8/23) of controls. However, the relative values of V-P differed significantly between the patient and control groups (−4.74±13.66 vs. 7.26±11.11, p=0.003). Only 26.1% (6/23) of the control subjects showed a PIQ>VIQ discrepancy, while the other 73.9% (17/23) had a VIQ>PIQ discrepancy. In comparison, 73.7% (14/19) of BECTS patients exhibited a PIQ>VIQ discrepancy, while the other 26.3% (5/19) had a VIQ>PIQ discrepancy (p=0.002) (Fig. 1A). The scores for the four factorial subscales of K-WISC-III (VCI, POI, FDI, and processing speed) were also lower in BECTS patients (p<0.001). Within the patient group, the subgroup of eight subjects with frequent EDs showed no significant differences in VIQ, PIQ, or FSIQ, but their VCI was relatively lower than for the rest of the group (84.38±14.05 vs. 97.82±14.99, p=0.064) (Fig. 1B).
The verbal and visuospatial memory performance scores determined using the AVLT and RCFT did not differ significantly between the patient and control groups. There were also no significant differences in the frontal executive function test scores between the patient and control groups.
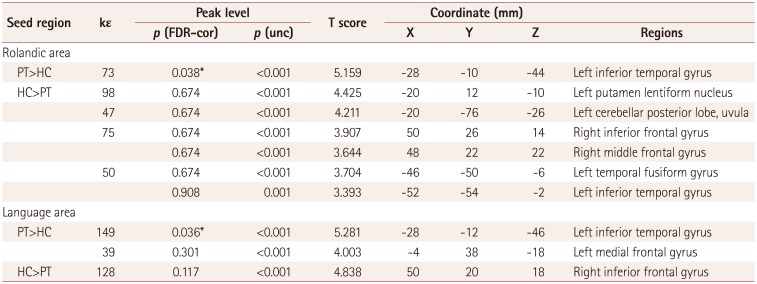
In the patient group, maps of the Rolandic FC exhibited positive correlations with more-restricted regions, including the bilateral superior temporal gyri and the right claustrum. In the control group, the Rolandic area exhibited strong positive FC correlations with distributed areas, including the left superior temporal gyrus, left middle frontal gyrus, and right superior frontal gyrus (Supplementary Fig. 1A in the online-only Data Supplement). The statistically significant voxel distributions in specific brain areas are summarized in Supplementary Table 1 (in the online-only Data Supplement).
In the patient group, the language area exhibited strong FC to distributed regions including the bilateral middle temporal gyri and the right occipital fusiform gyrus. In the control group, the language area exhibited strong positive FC correlations with distributed areas, including the left supramarginal gyrus and bilateral cerebellar pyramis (Supplementary Fig. 1B in the online-only Data Supplement). Voxel distributions in specific brain areas with statistical significance are also summarized in Supplementary Table 1 (in the online-only Data Supplement).
In our subjects, either the Rolandic or language FC to voxels with significant group differences in two-sample t-tests demonstrated significant negative correlations with scores on the K-WISC-III IQ subscales, especially those related to language domains. More specifically, enhanced Rolandic FC to voxels in the left inferior temporal gyrus was correlated with more impaired performance as measured by VIQ (r=−0.501, p=0.0007) (Fig. 3A) and FSIQ (r=−0.472, p=0.0016) (Fig. 3C). PIQ showed a trend of being negatively correlated with the Rolandic FC to the same area, but this was not statistically significant after correcting for multiple comparisons (r=−0.330, p=0.0329) (Fig. 3B). A higher language FC to voxels in the left inferior temporal gyrus was correlated with lower performance as measured by VIQ (r=−0.541, p=0.0002) (Fig. 4A) and FSIQ (r=−0.526, p=0.0003) (Fig. 4C). PIQ also exhibited a trend of being negatively correlated with the language FC to the same area, but this was not statistically significant after correcting for multiple comparisons (r=−0.400, p=0.0086) (Fig. 4B). However, there was no statistical significance in the BECTS group alone without control subjects in the correlation analysis.
Correlation plots of factorial subscales with either the Rolandic or language FC to the same area are presented in Supplementary Figs. 2 and 3 (in the online-only Data Supplement), respectively. Enhanced Rolandic FC to voxels with significant group differences was correlated with greater impairments in cognitive performance as measured by VCI (r=−0.474, p=0.0015) (Supplementary Fig. 2A in the online-only Data Supplement), FDI (r=−0.434, p=0.0041) (Supplementary Fig. 2C in the online-only Data Supplement), and processing speed (r=−0.506, p=0.0006) (Supplementary Fig. 2D in the online-only Data Supplement). Enhanced language FC to voxels with significant group differences was correlated with greater impairment of cognitive performance as measured by VCI (r=−0.517, p=0.0005) (Supplementary Fig. 3A in the online-only Data Supplement) and FDI (r=−0.438, p=0.0037) (Supplementary Fig. 3C in the online-only Data Supplement).
The present study investigated intellectual functioning in various cognitive domains in BECTS patients and control subjects, as well as their relationships with FC changes in restingstate brain networks using Rolandic and language FC analysis. The main aim was to provide new insights into the biological mechanisms underlying BECTS. The main findings of this study were as follows: 1) BECTS patients showed significantly lower cognitive performance as measured by K-WISC-III IQ scores, in addition to different V-P patterns, 2) both the Rolandic and language areas exhibited greater FC to voxels in the left inferior temporal gyrus in BECTS patients than in healthy controls, and 3) the K-WISC-III IQ scores of our subjects were negatively correlated either with Rolandic or language FC to voxels in the left inferior temporal gyrus, demonstrating higher seed-based FC values in BECTS patients than in the healthy controls.
Recent studies of BECTS have addressed various functional deficits including in language, attention, memory, and executive functioning, in addition to academic achievements.4141516212336 Our BECTS patients also showed significantly worse performance across various measures of intelligence in K-WISC-III tests; however, the verbal and visuospatial memory performance scores and frontal executive function test scores did not differ significantly between the patient and control groups, which is not fully consistent with the findings of previous studies. Nevertheless, some of our findings are compatible with the previous literature, such as our BECTS patients exhibiting prominently lower performance in the language-related cognitive domain. The VIQ (among IQ measurement parameters) and the VCI (among factorial subscales) showed the largest mean differences between BECTS patients and healthy controls. We also noted differences in the V-P pattern between the BECTS and controls, with the patient group showing a much higher proportion with a PIQ>VIQ discrepancy than the controls. Regarding language dysfunction in BECTS, many previous studies have found abnormal literacy and language function,16233738 and a recent meta-analysis emphasized the presence of reading and phonological processing deficits and highlighted the importance of early literacy and language assessment in BECTS.21
A particularly interestingly finding of the present study was that our patient subgroup with frequent EDs showed relatively lower scores on the VCI subscale, which is the most language-specific cognitive measurement. Since our study had a cross-sectional design, it is difficult to determine whether these cognitive differences or FC alterations result from the trajectory of neurodevelopmental disruption or secondary pathology induced by the propagation of EDs. However, the negative tendency of the VCI score combined with the ED frequency suggests that poor seizure control has a deleterious impact on cognitive function.2339
Most of our patients were taking AEDs, which could reduce both neuronal excitability and irritability, and so possibly impair cognitive functioning including the psychomotor processing speed, sustained attention (i.e., vigilance), and learning.4041 In general, polypharmacy, increasing the AED dosage, and taking older agents are known to be associated with cognitive toxicity. However, our patients were receiving standard monotherapy dosages, with the exception of two cases of AED switching, and none of the patients were taking AEDs that are well known to exert adverse effects on cognition, such as barbiturates, benzodiazepines, and topiramate.
Several previous studies have documented that BECTS alters FC between motor and language networks during word-generation tasks or in the resting state.15161718192037 In our rs-fMRI investigation of the difference in seed-based FC patterns in BECTS compared with healthy controls, one of our seed regions was the Rolandic area because BECTS patients usually exhibit characteristic facial or arm sensorimotor seizures in accordance with EEG findings, indicating the Rolandic cortex as a putative source of EDs.42 The present regional patterns of positive Rolandic FC in the patient and control groups were similar to previous observations of aberrant FC between the motor and language-related brain networks,1617 and decreased FC in the default mode network, bilateral occipital lobes, and bilateral orbitofrontal cortex.182022 The Rolandic ROIs exhibited greater FC to voxels in the left inferior temporal gyrus in BECTS patients than in control subjects, and this region is related to the semantic processing of spoken words; analogous results were obtained for language FC.
The core language network has been well documented,31323334 and our findings are also concordant with recent rs-fMRI studies of BECTS suggesting that abnormally enhanced connectivity patterns can interrupt the normal language network and its function.18 Task-aided fMRI studies have also revealed that BECTS patients adopt less-efficient cognitive strategies, relying on regions outside of the core language network to perform a given task, such as using visual areas to make semantic decisions, whereas healthy controls employed regions related to attention and response monitoring (e.g., cingulate cortex).1516172237 However, as we investigated the resting-state brain networks of BECTS patients, we did not find specific BOLD fluctuations related to language processing, including reading, phonological processing, and expressive or receptive language.
To determine whether these FC alterations are correlated with cognitive impairments, we plotted the cognitive test results against the seed-based FC values. The correlation analysis showed significant negative correlations between the Rolandic or language FC to the left temporal gyrus and K-WISC-III test scores, especially for the verbal subdomains. These findings suggest that the connectivity could be enhanced in the language-related areas to compensate for poor verbal cognition. However, our correlation analysis did not produce statistically significant results for the patient group alone (i.e., without controls), possibly due to the left temporal gyrus participating in semantic processing in both groups. Nevertheless, since the FC values to voxels of those regions differed significantly between the BECTS patients and control subjects, further studies involving large numbers of subjects are needed to evaluate whether there are intergroup differences in the correlation coefficients. We also could not determine the effects of the location or frequency of EDs on the correlation coefficient of FC to verbal cognition. Longitudinal studies would be helpful for disentangling this, and explaining whether the observed abnormalities represent a temporary delay in brain maturation or a persistent deviation from the normal developmental trajectory.
In summary, we suggest that the Rolandic spike focus interrupts the normal neuronal connectivity of the language network in the developing brain through either aberrant enhancement or disruption. In addition to our previous data showing neuroimaging abnormalities in DTI and VBM,58 the current rs-fMRI study has demonstrated differences in FC in the language and Rolandic areas between BECTS patients and healthy controls. We also found that our BECTS patients showed impaired cognitive performance, especially in the verbal domain, and correlated this with FC alterations in resting-state language-related brain networks. These observations suggest that altered FC in these cortical networks could be responsible for the cognitive dysfunction in these subjects.
Acknowledgements
This study was supported by the grant of Samsung Biomedical Research Institute to J.H. Lee, and supported by the grants of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015R1C1A1A01052438 to C.H. Park, and 2014R1A2A1A11052103 to H.W. Lee), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI14C1989) to H.W. Lee.
References
1. Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012; 79:1384–1391. PMID: 22972641.

2. Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav. 2011; 20:550–555. PMID: 21315660.

3. Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008; 49:608–614. PMID: 18070088.

4. Filippini M, Ardu E, Stefanelli S, Boni A, Gobbi G, Benso F. Neuropsychological profile in new-onset benign epilepsy with centrotemporal spikes (BECTS): focusing on executive functions. Epilepsy Behav. 2016; 54:71–79. PMID: 26667848.

5. Lee JH, Kim SE, Park CH, Yoo JH, Lee HW. Gray and white matter volumes and cognitive dysfunction in drug-naïve newly diagnosed pediatric epilepsy. Biomed Res Int. 2015; 2015:923861. PMID: 26417604.

6. Bhise VV, Burack GD, Mandelbaum DE. Baseline cognition, behavior, and motor skills in children with new-onset, idiopathic epilepsy. Dev Med Child Neurol. 2010; 52:22–26. PMID: 19702836.

7. Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009; 73:526–534. PMID: 19675309.

8. Kim SE, Lee JH, Chung HK, Lim SM, Lee HW. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur J Neurol. 2014; 21:708–717. PMID: 24330132.

9. Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016; 57:1546–1557. PMID: 27554793.

10. Tracy JI, Doucet GE. Resting-state functional connectivity in epilepsy: growing relevance for clinical decision making. Curr Opin Neurol. 2015; 28:158–165. PMID: 25734954.
11. Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009; 213:525–533. PMID: 19565262.

13. Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain. 2008; 131:2264–2286. PMID: 18718967.

14. Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008; 49:1570–1580. PMID: 18410358.

15. Datta AN, Oser N, Bauder F, Maier O, Martin F, Ramelli GP, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013; 54:487–494. PMID: 23297860.

16. Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009; 50:2276–2284. PMID: 19292755.

17. Besseling RM, Overvliet GM, Jansen JF, van der Kruijs SJ, Vles JS, Ebus SC, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013; 107:253–262. PMID: 24210960.

18. Zeng H, Ramos CG, Nair VA, Hu Y, Liao J, La C, et al. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): a resting state fMRI study. Epilepsy Res. 2015; 116:79–85. PMID: 26354170.

19. Xiao F, Li L, An D, Lei D, Tang Y, Yang T, et al. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): a resting-state fMRI study. Epilepsy Behav. 2015; 45:234–241. PMID: 25825370.

20. Tang YL, Ji GJ, Yu Y, Wang J, Wang ZJ, Zang YF, et al. Altered regional homogeneity in rolandic epilepsy: a resting-state FMRI study. Biomed Res Int. 2014; 2014:960395. PMID: 25247197.

21. Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015; 57:1019–1026. PMID: 26219529.

22. Oser N, Hubacher M, Specht K, Datta AN, Weber P, Penner IK. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy Behav. 2014; 33:12–17. PMID: 24583653.

23. Riva D, Vago C, Franceschetti S, Pantaleoni C, D'Arrigo S, Granata T, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2007; 10:278–285. PMID: 17267289.

24. Engel J Jr. International League Against Epilepsy (ILAE). A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001; 42:796–803. PMID: 11422340.

25. Engel J Jr. Classification of epileptic disorders. Epilepsia. 2001; 42:316. PMID: 11442146.
26. Kwak KJ, Park HW, Kim CT. A study for the standardization of Korean WISC-3(1). Korean J Ind Organ Psychol. 2002; 15:19–33.
27. Vakil E, Greenstein Y, Blachstein H. Normative data for composite scores for children and adults derived from the Rey Auditory Verbal Learning Test. Clin Neuropsychol. 2010; 24:662–677. PMID: 20155574.

28. Kirkwood MW, Weiler MD, Bernstein JH, Forbes PW, Waber DP. Sources of poor performance on the Rey-Osterrieth Complex Figure Test among children with learning difficulties: a dynamic assessment approach. Clin Neuropsychol. 2001; 15:345–356. PMID: 11778773.

29. Lee JB, Kim JS, Seo WS, Shin HJ, Bai DS, Lee HR. The validity and reliability of ‘Computerized Neurocognitive Function Test’ in the elementary school child. Korean J Psychosom Med. 2003; 11:97–117.
30. Shin MS, Park MJ. Stroop Color and Word Test. Seoul: Hakjisa;2007.
31. Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin. 2014; 4:374–382. PMID: 24567909.

32. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012; 22:158–165. PMID: 21616982.

33. Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010; 1191:62–88. PMID: 20392276.

34. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009; 106:13040–13045. PMID: 19620724.

35. Wechsler D. WISC-III: Wechsler Intelligence Scale for Children. San Antonio: Psychological Corporation;1991.
36. Verrotti A, Filippini M, Matricardi S, Agostinelli MF, Gobbi G. Memory impairment and benign epilepsy with centrotemporal spike (BECTS): a growing suspicion. Brain Cogn. 2014; 84:123–131. PMID: 24362071.

37. Vannest J, Szaflarski JP, Eaton KP, Henkel DM, Morita D, Glauser TA, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol. 2013; 28:435–445. PMID: 22761402.

38. Papavasiliou A, Mattheou D, Bazigou H, Kotsalis C, Paraskevoulakos E. Written language skills in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2005; 6:50–58. PMID: 15652734.

39. Xiao F, An D, Lei D, Li L, Chen S, Wu X, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy: An EEG-fMRI study. Neurology. 2016; 86:544–551. PMID: 26747882.
40. Bromley RL, Leeman BA, Baker GA, Meador KJ. Cognitive and neurodevelopmental effects of antiepileptic drugs. Epilepsy Behav. 2011; 22:9–16. PMID: 21684214.

41. Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011; 4:385–407. PMID: 22164192.

42. Kellaway P. The electroencephalographic features of benign centrotemporal (rolandic) epilepsy of childhood. Epilepsia. 2000; 41:1053–1056. PMID: 10961638.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.1.48.
Supplementary Fig. 1
Within-group maps of the positive Rolandic and language FC in the BECTS patient and control groups. A: In the patient group, maps of the Rolandic FC exhibited positive correlation to more-restricted regions, including the bilateral superior temporal gyri and the right claustrum. In the control group, the Rolandic area exhibited strong positive FC correlations with distributed areas, including the left superior temporal gyrus, left middle frontal gyrus, and right superior frontal gyrus. B: In the patient group, maps of the language FC exhibited positive correlation to more-restricted regions, including the bilateral middle temporal gyri and the right occipital fusiform gyrus. In the control group, the language area exhibited strong positive FC correlations with distributed areas, including the left supramarginal gyrus and bilateral cerebellar pyramis. Statistically significant voxel distributions in specific brain areas are summarized in Supplementary Table 1 (in the online-only Data Supplement). BECTS: benign childhood epilepsy with centrotemporal spikes, FC: functional connectivity.
Supplementary Table 1
Regions exhibiting positive seed-based functional connectivity within the benign childhood epilepsy with centrotemporal spikes patient and control groups
Supplementary Fig. 2
Relationship between scores on the factorial subscales of the Korean version of the Wechsler Intelligence Scale for Children-III and Rolandic FC. Rolandic FC to voxels in the left inferior temporal gyrus, which was greater in the patient group (red dots) than in the control group (blue squares), was correlated with greater impairment of cognitive performance as measured by the (A) VCI (r=−0.474, p=0.0015), (B) POI (r=−0.198, p=0.2097), (C) FDI (r=−0.434, p=0.0041), and (D) processing speed (r=−0.506, p=0.0006). The cutoff for statistical significance was a corrected p of 0.0071. FC: functional connectivity, FDI: freedom from distractibility index, POI: perceptual organization index, VCI: verbal comprehension index.
Supplementary Fig. 3
Relationship between Korean version of the Wechsler Intelligence Scale for Children-III factorial subscales and language FC. Language FC to voxels in the left inferior temporal gyrus, which was greater in the patient group (red dots) than in the control group (blue squares), was correlated with greater impairment of cognitive performance as measured by (A) VCI (r=−0.517, p=0.0005), (B) POI (r=−0.343, p=0.0261), (C) FDI (r=−0.438, p=0.0037), and (D) processing speed (r=−0.320, p=0.0389). The cutoff for statistical significance was a corrected p of 0.0071. FC: functional connectivity, FDI: freedom from distractibility index, POI: perceptual organization index, VCI: verbal comprehension index.
Fig. 1
Group differences in the cognitive performance between BECTS patients and control subjects. A: The control group showed a higher proportion of subjects with a VIQ>PIQ discrepancy, whereas the patient group contained a significantly higher percentage of those with a PIQ>VIQ discrepancy (p=0.002). B: The eight frequent (>1 per minute) ED presenters in the patient group exhibited a lower VCI than the infrequent ED presenters (p=0.064). BECTS: benign childhood epilepsy with centrotemporal spikes, ED: epileptiform discharge, PIQ: performance intelligence quotient, VCI: verbal comprehension index, VIQ: verbal intelligence quotient.

Fig. 2
Group differences in the seed-based FC patterns between BECTS patients and control subjects. A: Increased FC was observed with voxels in the left inferior temporal gyrus in the patient group than in the control group when using a Rolandic mask covering the bilateral inferior frontal gyri (pars triangularis and pars opercularis) and the Rolandic operculum and insula as the seed. B: Increased FC was observed with voxels in the left inferior temporal gyrus in the patient group than in the control group when using a language mask covering the bilateral inferior frontal gyri, middle and superior temporal gyri, and supramarginal gyri in addition to the left angular gyrus and left cerebellar crus I as the seed. BECTS: benign childhood epilepsy with centrotemporal spikes, FC: functional connectivity.
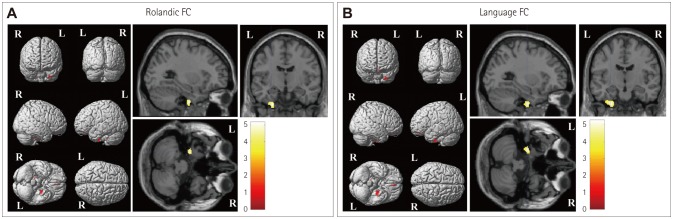
Fig. 3
Relationship between intelligence quotient scores on the Korean version of the Wechsler Intelligence Scale for Children-III and Rolandic FC. Rolandic FC to voxels in the left inferior temporal gyrus, which was greater in the patient group (red dots) than the control group (blue squares), was correlated with greater impairment of cognitive performance as measured by (A) VIQ (r=−0.501, p=0.0007), (B) PIO (r=−0.330, p=0.0329), and (C) FSIQ (r=−0.472, p=0.0016). The cutoff for statistical significance was a corrected p of 0.0071. FC: functional connectivity, FSIQ: full-scale intelligence quotient, PIQ: performance intelligence quotient, VIQ: verbal intelligence quotient.
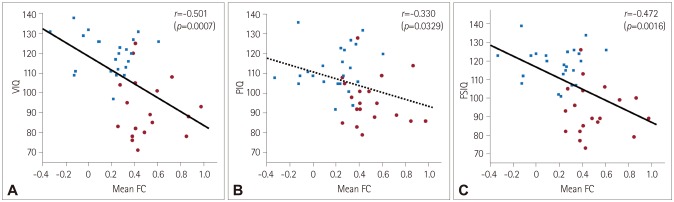
Fig. 4
Relationship between Korean version of the Wechsler Intelligence Scale for Children-III intelligence quotient scores and language FC. Language FC to voxels in the left inferior temporal gyrus, which was greater in the patient group (red dots) than in the control group (blue squares), was correlated with greater impairment of cognitive performance as measured by (A) VIQ (r=−0.541, p=0.0002), (B) PIQ (r=−0.400, p=0.0086), and (C) FSIQ (r=−0.526, p=0.0003). The cutoff for statistical significance was a corrected p of 0.0071. FC: functional connectivity, FSIQ: full-scale intelligence quotient, PIQ: performance intelligence quotient, VIQ: verbal intelligence quotient.
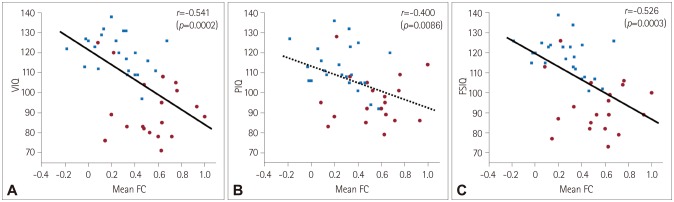
Table 1
Clinical characteristics of the BECTS patients and healthy controls
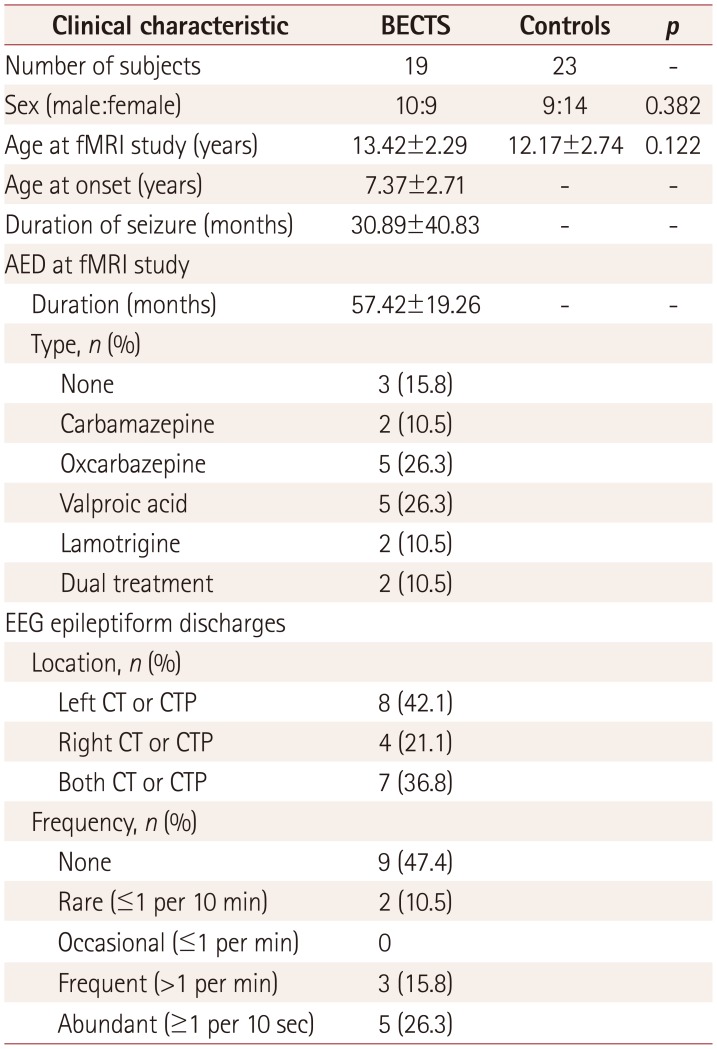
Table 2
Comparisons of neuropsychological test scores between the BECTS patients and healthy controls
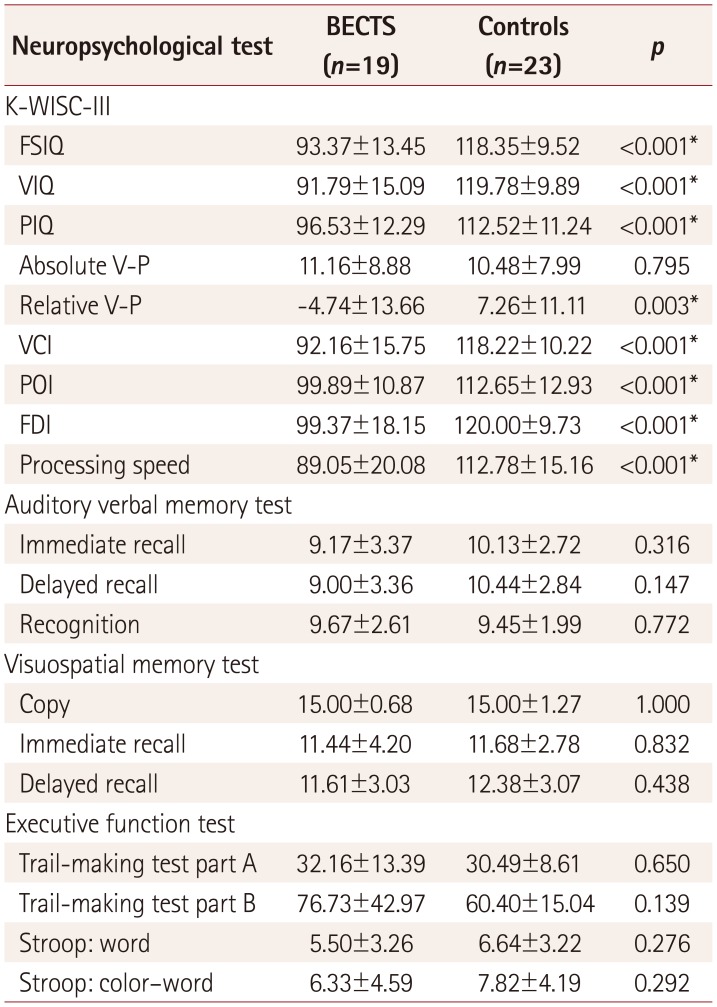
Data are mean±standard-deviation values.
*Statistical significance at p<0.05.
BECTS: benign childhood epilepsy with centrotemporal spikes, FDI: freedom from distractibility index, FSIQ: full-scale intelligence quotient, K-WISC-III: Korean version of the Wechsler Intelligence Scale for Children-III, PIQ: performance intelligence quotient, POI: perceptual organization index, VCI: verbal comprehension index, VIQ: verbal intelligence quotient, V-P: verbal-performance intelligence quotient discrepancy.
Table 3
Regions exhibiting differences between the patient and control groups in seed-based functional connectivity to voxels throughout the brain




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download