Abstract
The authors report a rare variation of the coeliac trunk, renal and testicular vasculature in a 27-year-old male cadaver. In the present case, the coeliac trunk and superior mesenteric artery was replaced by a modified coeliacomesenteric trunk formed by hepato-gastric and superior mesenteric arteries. Here the hepato-gastric artery or trunk contributed towards the total hepatic inflow as well as a gastro-duodenal artery. A separate right gastric artery and an additional superior pancreatico-duodenal artery was also found in addition with a retro-aortic left renal vein and a bilateral double renal arterial supply. The aforementioned coeliac trunk variation, to our knowledge, has never been reported before and this variation combined with the renal vasculature requires careful surgical consideration.
The typical trifurcation of the coeliac trunk into the left gastric, common hepatic and splenic arteries was first described by Albrecht von Haller (1708–1777) [1]. Haller, born in Berne, Switzerland, studied medicine at University of Tübingen in 1723. Haller felt dissatisfied with his progress at Tübingen and became a student of Boerhaave and Albinus at Leyden University in 1727. The young prodigy with a wide-ranging intellect received his medical degree at 19 years old [2]. The coeliac branches (tripos Halleri) represents the normal configuration of the blood supply of the foregut viscera within the abdominal cavity. Knowledge of any deviation from the norm, as a result embryologic changes in the development of the ventral splanchnic arteries, proves indispensable in the planning and execution surgical operations. This holds for procedures such as gastric resection, partial hepatectomy, pancreatico-duodenectomy, laparoscopic surgeries, and traumatic injuries to the abdomen [3].
An anatomical variation in the coeliac trunk, renal and testicular vasculature was observed during routine dissection of the abdominal region of a 27-year-old male cadaver from the Western Cape, South Africa. The formalin embalmed cadaver formed part of a cohort of ten (3 female, 7 male), kindly donated to the Department of Anatomy, School of Medicine, University of Namibia, Namibia.
The cause of death was documented as pulmonary tuberculosis with no abdominal pathologies of note. Upon dissection it was found that the mesenteric artery originated from the coeliac artery at T12 (Fig. 1A, B). The coeliacomesenteric trunk presents with a hepato-gastric trunk (circa 2.3 cm in length), splenic artery on the left, and a stand-alone right gastric artery (Fig. 1A, B). The latter supplied not only the lesser curvature of the stomach but also the head of the pancreas and superior part of the duodenum; forming a secondary pancreatico-duodenal supply. Of interest was the hepatogastric trunk further divided into a common hepatic artery (Fig. 1A, B). This in turn divided into a left and right hepatic (with cystic artery) arteries at the porta hepatis (Fig. 1A, B). The gastro-duodenal artery originated from the right hepatic and then descended to the duodenum and pancreas; forming a primary superior pancreatico-duodenal supply (Fig. 1A, B). The inferior pancreatico-duodenal arteries followed its traditional origin form the superior mesenteric arty (Fig. 1A, B).
Additional findings in the same cadaver included a bifurcation of the abdominal aorta at L3 and a bilateral double renal arterial supply (Fig. 2A, B). The right pair of renal arteries originated at L1 compared to the left sided pair (L1 and L2) (Fig. 2A, B). The left kidney was slightly enlarged and more inferior, circa 2.5 cm, compared to the right kidney. The left testicular artery originated from the inferior renal artery and this pattern repeated itself on the right (Fig. 2A, B). Of interest was that the right testicular artery passed posterior of the inferior vena cava (Fig. 2A, B). The left renal vein was found to be retro-aortic and received venous blood from the left lumbar veins, posterior of the aorta. The left and right testicular veins drained into the left renal vein and inferior vena cava respectively (Fig. 2A, B). The inferior mesenteric artery when observed, was normal and no other variations were observed.
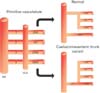
The coeliac trunk is the primary anterior unpaired artery from the abdominal aorta supplying structures with their embryological origin in the foregut. The standard “textbook” description sees three major branches from the coeliac trunk; left gastric, splenic and common hepatic [4]. There are variations in the branches of the coeliac trunk and the work by Sumaltha et al. serves as reference, however there are rare anomalies where it is completely absent in some individuals [45]. Variations in the anatomy of the coeliac trunk is ascribed to the development of the embryonic dorsal aortae. The dorsal aorta can be subdivided into various branches including the ventral (visceral), segmental branches, which eventually develop to form the coeliac trunk and becomes the artery of the foregut. The superior mesenteric artery and inferior mesenteric artery also originate from the branches of the dorsal aorta. It is important to note that the arteries' level of origin changes as the gut elongates and descends; the arteries also descend [67]. Four primitive splanchnic branches, which arise from the developing abdominal aorta, are connected by the ventral longitudinal anastomosis (Lang's anastomosis) (Fig. 3). The central two splanchnic branches disappear and the first and fourth roots are merged by a longitudinal anastomosis, which forms the classical trifurcation of the coeliac trunk. The longitudinal anastomosis then disappears (Fig. 3) [6]. The persistence of vessels, or their components, that normally disappear or the disappearance of vessels that normally persist, can lead to various vascular variations (Fig. 3) [8]. One such variation is a common hepato-gastric trunk and this has previously been discussed, with the left gastric artery arising from the common hepatic artery and the splenic artery originating from the abdominal aorta or from a splenomesenteric trunk [359]. These reports are unlike our case, where the splenic artery originates from a combined hepatogastric and mesenteric arterial trunk. Also, this is the first case, to our knowledge, reporting the origin of the right gastric artery from the aforementioned combined arterial trunk.
Retro-aortic renal veins are uncommon variations in the renal venous drainage, although many variations have been reported, these are likely to be under reported, given that many do not usually present with pathological manifestations [10]. The retro-aortic variation in renal venous drainage has been classified into four categories; type I is seen in our case [11]. In type I the dorsal retroaortic limb of the left renal vein persists and joins the inferior vena cava after obliteration of the ventral preaortic limb. Type II follows a similar origin but the dorsal limb transforms into a retro-aortic variation at a level of L4 to L5. Type III typically presents with a circumaortic left renal vein, i.e., an anterior and poster venous collar. Lastly, in type IV the ventral preaortic limb of the left renal vein is obliterated. The dorsal limb forms the retro-aortic vein, as in types I and II, but the vein runs obliquely and joins the left common iliac vein caudally [11]. The development of this variation is linked to the embryological development of the inferior vena cava (IVC). The IVC develops from a network of three pairs of communication veins, namely: the posterior cardinal veins; the subcardinal veins; and the supracardinal veins. Anastomotic communications link the subcardinal and supracardinal channels and this in turn forms a collar veins that encircles the aorta. The ventral portion normally persists as the retro-aortic variation arises from the dorsal portion [12]. These cases may present with microscopic haematuria, occasionally varicocele and left flank pain due to compression of the left renal vein by the aorta. The compression of the left renal vein is most commonly described and seen as it passes between the aorta and the origin of the superior mesenteric artery, giving the typical “nutcracker phenomenon,” which may result in the “nutcracker syndrome” when clinical signs manifest. The retro-aortic renal vein compression is usually referred to as “posterior nutcracker syndrome” [1314]. The clinical signs of both syndromes are similar and imaging would be the only ante-mortem method to diagnose the variation prior to surgery. The description in our case of the slightly enlarged left kidney is indicative of reduced venous return from the left kidney to the inferior vena cava, however the diameter of the ureter was not different from the right.
The unique combination of novel presentations and rare variations in this case reiterate a need for a comprehensive understanding of anatomy of the coeliac trunk and the structures supplied by it. These variations have huge significance in abdominal surgery planning, particularly pertinent to renal, hepatic and gastric surgeries and aortic repairs.
Figures and Tables
Fig. 1
(A) A cadaveric dissection demonstrating the coeliacomesenteric trunk with the hepato-gastric and superior mesenteric arteries. The left gastric and common hepatic arteries originated from the former. The common hepatic artery (dashed red arrow) continued to form the proper hepatic artery which in turn gave rise to the left and right hepatic arteries. The cystic artery originated from the right hepatic artery and gastro-duodenal artery descended towards the inferior (dashed red arrow). The right gastric artery was a stand-alone branch of the coeliacomesenteric trunk. The splenic artery continued its normal course. (B) A sketch demonstrating the coeliacomesenteric variation with the hepato-gastric and superior mesenteric arteries. The pancreatico-duodenal arterial supply originated from the gastro-duodenal artery, and right gastric and superior mesenteric arteries. The asterisk denotes a secondary superior pancreatico-duodenal and supraduodenal arterial supply. Scale bar=1 cm. AA, abdominal aorta; CA, cystic artery; CD, cystic duct; CHD, common hepatic duct; FL, Falciform ligament; GDA, gastro-duodenal artery; HGA, hepato-gastric artery; IPD, inferior pancreatico-duodenal artery; IVC, inferior vena cava; LGA, left gastric artery; LHA, left hepatic artery; LK, left kidney; LLL, left liver lobe; P, pancreas; PHA, proper hepatic artery; PV, portal vein; RGA, right gastric artery; RHA, right hepatic artery; RK, right kidney; RLL, right liver lobe; SA, splenic artery; SMA, superior mesenteric artery; SPD, superior pancreatico-duodenal artery.

Fig. 2
(A, B) A cadaveric dissection and artistic representation, demonstrating the renal and testicular vasculature of the same individual. The abdominal aorta gave rise to a double pair, left and right, renal arteries. The left kidney was located more inferiorly compared to the right kidney. Both ureters, left and right, were normal. The retro-aortic left renal vein can be seen moving posterior (dashed blue arrow in panel A and phantom lines in panel B) to the abdominal aorta. The testicular arteries, asterisks in panel A, are indicated as the right and left testicular arteries in panel B. The right testicular artery was found posterior of the inferior vena cava but both the left and right testicular veins followed their normal course. Scale bar=1 cm. AA, abdominal aorta; IMA, inferior mesenteric artery; IVC, inferior vena cava; LK, left kidney; LRA, left renal artery; LTA, left testicular artery; LTV, left testicular vein; LU, left ureter; RA LRV, retro-aortic left renal vein; RK, right kidney; RRA, right renal artery; RTA, right testicular artery; RTV, right testicular vein; RU, right ureter.

Fig. 3
An illustration showing the embryological development of the coeliac artery and superior mesenteric artery. The diagram on the left depicts the four primitive splanchnic branches (numbered), originating from the dorsal aorta and a ventral longitudinal anastomosis (Lang's anastomosis). The central two splanchnic branches normally disappear and the longitudinal anastomosis closes between the third and fourth root to form a typical coeliac trunk (top right). The persistence of the ventral longitudinal anastomosis and regression of the first or fourth root (bottom right) can lead to the formation of a coeliacomesenteric trunk. DA, dorsal aorta; CHA, common hepatic artery; CMT, coeliacomesenteric trunk; LGA, left gastric artery; SA, splenic artery; SMA, superior mesenteric artery; VLA, ventral longitudinal anastomosis.
References
1. Haller VA. Icones anatomicae quibus praecipuae aliquae partes corporis humani delineatae proponuntur et arteriarum potissimum historia continetur. Gottingen: A Vandenhoeck;1756.
2. Bay JC. Albrecht Von Haller Medical Encyclopedist. Bull Med Libr Assoc. 1960; 48:393–403.
3. Mariani GA, Maroni L, Bianchi L, Broccoli A, Lazzarini E, Marchegiani G, Mazzotti A, Mazzotti MC, Billi AM, Piccari GG, Cocco L, Manzoli L. Hepato-gastric and spleno-mesenteric arterial trunks: anatomical variation report and review of literature. Ital J Anat Embryol. 2013; 118:217–222.
4. Sumalatha S, Hosapatna M, Bhat KR, D'Souza AS, Kiruba L, Kotian SR. Multiple variations in the branches of the coeliac trunk. Anat Cell Biol. 2015; 48:147–150.

5. Fahmy D, Sadek H. A case of absent celiac trunk: case report and review of the literature. Egypt J Radiol Nucl Med. 2015; 46:1021–1024.

6. Lovisetto F, Finocchiaro De Lorenzi G, Stancampiano P, Corradini C, De Cesare F, Geraci O, Manzi M, Arceci F. Thrombosis of celiacomesenteric trunk: report of a case. World J Gastroenterol. 2012; 18:3917–3920.

7. Walker TG. Mesenteric vasculature and collateral pathways. Semin Intervent Radiol. 2009; 26:167–174.

8. Tandler J. Über die varietaten der arteria coeliaca und deren entwickelung. Anat Hefte. 1904; 25:475–500.
9. Fiorello B, Corsetti R. Splenic artery originating from the superior mesenteric artery: an unusual but important anatomic variant. Ochsner J. 2015; 15:476–478.
10. Hayashi M, Kume T, Nihira H. Abnormalities of renal venous system and unexplained renal hematuria. J Urol. 1980; 124:12–16.

11. Nam JK, Park SW, Lee SD, Chung MK. The clinical significance of a retroaortic left renal vein. Korean J Urol. 2010; 51:276–280.

12. Mathews R, Smith PA, Fishman EK, Marshall FF. Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology. 1999; 53:873–880.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download