Abstract
Cimetidine is an H2 receptor antagonist that has an antiandrogenic effect. It intervenes with the conversion of testosterone into estrogen in the Sertoli cells with accompanying testicular structural changes. In the present study, the microscopic and the ultrastructural changes induced by cimetidine and the effect of vitamin B12 as a protective agent on rat testes were studied. Immunoexpression of estrogen receptor β (ERβ) in testes was evaluated. Twenty-four adult male rats were divided into four groups: control, cimetidine-treated, vitamin B12 treated, and combined cimetidine and vitamin B12 treated. The experimental rats were administered with cimetidine and/or vitamin B12 for 52 days. Group II rats showed marked atrophy of the seminiferous tubules with a significant increase in tubular diameter and decrease in the tubular luminal and epithelial areas. Ultrastructure of this group showed irregular Sertoli cells with basal cytoplasmic vacuolation and significantly thickened basement membrane. ERβ immunoexpression was similar to controls. Group III rats showed near normal seminiferous tubular structures with minimal cellular alterations and the immunoreactivity of the testicular sections was very close to normal. However, group IV rats showed markedly immunopositive detached cells, spermatids, and primary spermatocytes. Cimetidine interferes with the control of spermatogenesis as evidenced by microscopic and ultrastructural studies and affection of ERβ receptors and vitamin B12 has a protective action against this harmful effect.
Cimetidine in commonly prescribed as H2 receptor antagonists for decreasing gastric acidity in cases of peptic ulcer and gastroesophageal reflux [1]. It acts by competing for H2 receptors in the gastric parietal cells. It has antitumor and anticancer properties because of its antiangiogenesis and anti-adhesion effects and its ability to inhibit tumor cell propagation and metastasis. It also has a hepatoprotective effect against hepatotoxins as isoniazide and rifampicin [2].
The main adverse effect of cimetidine is its antiandrogenic effect [3]. This effect occurs either by the competitive blocking of the hydrotestosterone receptors in the pituitary-hypothalamus axis and in the target tissues or by interference with the conversion of testosterone into estrogen in the Sertoli cells [4].
Testosterone is converted into estrogen in the seminiferous epithelium. This process is mediated by cytochrome P450 enzyme, which presents in both Sertoli and Leydig cells [45]. Two receptors mediate the action of estrogen on the reproductive system: estrogen receptor α (ERα) and estrogen receptor β (ERβ), of which, ERβ is more prevalent in the testis [67]. In experimental animals, cimetidine was found to decrease the weight of the testis and seminal vesicles and reduces the serum level of testosterone and follicle-stimulating hormone (FSH), and this effect is manifested by oligospermia, impotence, and loss of libido [48].
Another substantial adverse effect of the long-term and high dose treatment of cimetidine is the inhibition of vitamin B12 absorption as it requires acidic medium to be absorbed, which causes vitamin B12 deficiency [910]. In our bodies, vitamin B12 is essential in cell division as it stimulates DNA synthesis [9]. The testis, being one of the active tissues in mitosis is mainly affected by its deficiency [1112]. Experimentally, vitamin B12 deficiency caused the decreased weight of the testis with atrophy of the seminiferous tubules and aplasia of the sperms and spermatids [313].
Supplementation of vitamin B12 was reported to improve spermatogenesis in patients suffering oligospermia and to recover testicular tissues after intoxication [14]. In rodents, vitamin B12 protects against testicular damage caused by cimetidine [312]. It has been suggested that besides the antiandrogenic effect of cimetidine, other nutritional disorders caused by it has an impact on the testis.
In the present study, we propose to investigate the histopathological, ultrastructural changes in the seminiferous tubules of cimetidine treated rats focusing on Sertoli cell changes and morphometric measurements of the seminiferous epithelium. The effect of vitamin B12 administration with cimetidine will be evaluated and the immunoexpression of estrogen receptors (ERβ) in the testicular section will be studied and compared among all groups.
Twenty-four adult male and six adult female Sprague-Dawley rats (200–250 g) were used in the present study. Animals were housed at the animal care facility of Mansoura Experimental Research Center at Mansoura Faculty of Medicine. All protocols were followed as per the guidelines for the Care and Use of Laboratory Animals; Mansoura Faculty of Medicine-Institutional Research Board (MFM-IRB) approved the study. The males were divided into four groups (six animals each) in addition to the female group; the control group (group I), the cimetidine group (group II), the vitamin B12 group (group III) and the combined cimetidine and Vitamin B12 group (group IV). Groups II and IV animals received 50 mg cimetidine per kg body weight (Tagamet, SmithKline Beecham, London, UK) in an aqueous solution intraperitoneally daily for 52 days (the period of rat spermatogenic cycle) [4]. Group I animals received saline intraperitoneally for 52 days. Groups III and IV animals received 3 µg of vitamin B 12-hydroxocobalamin (Rubranova, 5,000 µg; Bristol-Myers Squibb) in the same aqueous solution with cimetidine for 52 days [3].
At the assigned time, the animals were fasted overnight and anesthetized with diethyl ether. After sacrifice, the right and left testes were separated from the adjacent epididymis and removed from the animal body. The testes were sectioned transversally, and specimens from the middle part of the testis were divided into two parts: one for the light microscopy and the other for electron microscopy. The uteri of the female rats were removed and sliced.
For light microscopy, the sections were immediately fixed in freshly prepared 10% neutral formaldehyde. Then, specimens were dehydrated in ascending grades of alcohol and embedded in paraffin and sectioned at 6 µm thickness and stained with hematoxylin and eosin (H&E). The uteri of the female rats were removed, sectioned and fixed in neutral formalin and embedded in paraffin to be used as positive control for the immunohistochemistry.
The testicular and the positive control uterine sections were hydrated and maintained for 20 minutes at 90℃ in citrate buffer (0.001 M, pH 6.0) to activate the antigen. For activation of the endogenous peroxide, the sections were immersed in 3% hydrogen peroxide for 10 minutes and washed in Tris-HCl 0.05 M; pH 7.5 buffer (TBS). For 14 hours, the sections were incubated with primary antibodies (rabbit polyclonal IgG anti-rat ERβ, Upstate Cell Signaling Solutions, Lake Placid, NY, USA), 1:200, diluted in TBS and 5% bovine serum albumin, at 4℃, then washed in TBS and incubated in solution containing biotinylated anti-rabbit antibodies (LSAB-plus kit, Dako Corporation, Carpinteria, CA, USA) for 30 minutes at room temperature, washed in TBS and incubated with streptavidin-peroxidase complex (LSAB-plus kit, Dako) for 15 minutes at room temperature, washed again in TBS and the reaction was exposed with 0,06% 3.3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Finally, sections were counterstained with hematoxylin, dehydrated and mounted in resin. To be sure that the immunoreaction could not have resulted from unspecific staining, testicular sections from the control group (group I), used as negative controls, were performed following the same protocol, except that the incubation in the primary antibody was replaced by incubation in non-immune serum [15].
For electron microscopy (EM), testicular sections were cut into small pieces and fixed in 4% glutaraldehyde in a phosphate buffer (pH 7.2) for 2–4 hours and post-fixed in 1% osmium tetraoxide. The samples were dehydrated and embedded in Spurr resin embedding medium. Semithin and ultrathin sections were obtained. Semithin sections were stained with toluidine blue. The ultrathin sections were mounted on copper grids and stained with uranyl acetate and lead citrate and observed with a Jeol 100 S transmission electron microscope(Jeol, Boston, USA) at Mansoura University, Egypt.
For morphometric assessment of seminiferous tubules, H&E slides were studied at 200× magnification and photographed using Olympus DP70 digital camera (Olympus Europe, Hamburg, Germany) attached to a light microscope (BX-51, Olympus) and Image Analyzer (Leica Q-Win standard, digital camera CH-9435 DFC 290, Wetzlar, Germany). In three non-serial sections, only the cross-sectioned seminiferous tubules with defined luminal border were studied. The shortest diameter, the total tubular area, the area of seminiferous epithelium and the luminal area of 20 tubules from each section with a total of 60 tubules per animal were considered for measurement.
The thickness of the basement membrane was subjectively graded (with the control taken as normal range); examination of the ultrathin sections as follows: grade 0, normal; grade 1, 25%–50% with diffuse fractionation; grade 2, 50%–75% with mild focal fractionation; and grade 3, >75% increase with diffuse fractionation [16].
For evaluation of the immunoreactivity, four non-serial testicular sections per animal from each group were estimated according to the intensity of ERβ immunolabeling. In each section, the ERβ-positive cells in six seminiferous tubules per section were analyzed according to the intensity of the ERβ immunoreactivity and scored as weak (+), moderate (++), or strong (+++) [17].
The morphometric measurements were reported as mean±standard deviation (SD). Instat-3 computer program (v2.04, GraphPad Software Inc., San Diego, CA, USA) was used to analyze the numbers and to evaluate the significant differences; the comparison of means between each two experimental groups was made using one-way ANOVA with post-hoc Tukey honest significant difference test. The differences were considered significant if P<0.05.
The means of the shortest diameter, the total tubular area, the area of seminiferous epithelium and the luminal area of the four groups are shown in the following Table 1 and Fig. 1. The shortest diameters of the seminiferous tubules were similar in the control group and group III. No statistical difference was observed between the two groups. A significant increase in the tubular diameter was observed in groups II and IV compared to the other groups.
The total tubular area and the area of the seminiferous epithelium in groups I and III were also close to each other with significant difference compared to rats received cimetidine in groups II and IV.
Ultrastructural analysis of the basement membrane of the seminiferous epithelium showed an increase in the thickness score of cimetidine-treated group (group II) and the cimetidine and vitamin B12 treated group (group IV) (grade 2/3 and 2 respectively) compared to the control and vitamin B12 only treated groups (grade 0 in both groups). The membrane appeared wavier, irregular with more deposition of collagen fibers compared to the control group.
The control group showed mild degree of immunoreactivity for ERβ in all layers of the germinal epithelium compared to the cimetidine-treated group (group II) which had a moderate to strong degree of reactivity. The third group had a mild degree of immune staining representation. The fourth group showed moderate degree of staining (Table 2).
Light microscopic examination of H&E sections of the testes of the control group revealed the normal testicular architecture with normal seminiferous tubular and germ cell layers and regular basement membrane. The stratified germinal epithelium is formed of different types of spermatogenic cells; spermatogonia, primary spermatocytes, spermatids, sperms, and Sertoli cells (Fig. 2A, B).
Group II (cimetidine-treated group) showed marked seminiferous tubular atrophy with epithelial disorganization and reduction in the thickness of the germinal epithelium (Fig. 2C). Most of the spermatocytes appeared with darkly stained pyknotic nuclei. Many vacuoles of variable sizes are noticed within the germinal epithelium. Severely affected tubules are lined by very few degenerating germinal epithelium and relatively wide empty lumina. The basement membrane is irregular with widened interstitial spaces containing congested blood vessels (Fig. 2D). Areas of loss of germ cells were also observed. Some detached germ cells were filling the tubular lumen. The Sertoli cells showed dislocation of the nuclei, which were irregular in shape and darkly stained with hematoxylin. Most nuclei were displaced away from the basement membrane (Fig. 2D).
Groups III (vitamin B12 treated group) and IV (cimetidine and vitamin B12 treated group) showed nearly normal histological structure of the seminiferous tubules and spermatogenesis. Some seminiferous tubules have organized lining epithelium with germ cells arranged in concentric layers and sperm tails in its lumen (Fig. 2E).
The positive control uterine sections revealed positive immunoreactivity in all layers of the uterus including the epithelium, endometrial glands and muscle tissue (Fig. 3A, B).
Testicular sections of control rats (group I) showed an evident cytoplasmic immunostaining for estrogen receptors (ERβ) which was observed in all layers of the germinal epithelium including the germ cells of the basal compartment (spermatogonia), primary spermatocytes and spermatids. An accentuated staining is observed in the elongating spermatids (Fig. 3C).
Although the germinal epithelium of group II rats showed evident vacuolations and disturbed pattern, the immunoreactivity was similar to control group. A strong immunostaining was observed in the detached germ cells filling the tubular lumen. The nuclei and cytoplasm of the spermatids and cytoplasm of the primary spermatocytes and germ cells of the basal compartment also showed also a strong immunopositivity. Some apoptotic cells with peripheral condensed chromatin were even positive to the immunoreactions (Fig. 3D).
Immunoreactivity of the testicular sections of rats treated with vitamin B12 (group III) rats was very close to that of the control group.
ERβ immunostaining in testicular sections of cimetidine and vitamin B12 treated rats (group IV) rats showed Sertoli cell vacuolations with variable degrees of cellular detachment in the tubular lumen. The detached cells showed positive immunoreactivity. The spermatids and primary spermatocytes' cytoplasm and nuclei were also immunopositive. Apoptotic cells with positive immunoreactions were less presented. The seminiferous tubules with maximal degree of destruction had strong immunoreactivity in the basal portion of their lining germ cells (Fig. 3E).
EM examination of the seminiferous tubules of the control group (group I) showed the Sertoli cells resting directly on the basement membrane. The apical part of its cytoplasm is extending from the basal lamina to the lumen of the seminiferous tubule and envelopes the adjacent germinal elements. The nucleus is oval shaped, situated close to the basement membrane and has an unfolded and sometimes has a very irregular shape; the nucleoplasm is homogenous with prominent nucleoli. The myoid cells appeared with delicate cytoplasm, and spindle-shaped nuclei lining the wall of the seminiferous tubules (Fig. 4A, B).
Group II rats (treated with cimetidine) showed massive changes in the Sertoli cells (Fig. 4C). The oval shape of the nuclei changed to be irregular. Most cells had basal vacuolations in the cytoplasm. Their nuclei lost their homogenesity. Lipid droplets were enlarged and widely distributed. The thickness of the basement membrane is increased (Fig. 4C). Remarkable vacuolations were seen in the cytoplasm of the cells especially in the basal between the nuclei and the basement membrane. Cytoplasmic fragments of the Sertoli cells were observed inbetween the germ cells near the lumen of the tubules with dark intercellular fragments and vacuolations.
Features of focal areas of spermatogenesis arrest at the spermatid level with degenerative changes in the germinal cells in the form of vacuolated cytoplasm and swollen mitochondria and thick, irregular tubular basement membrane (Fig. 4D).
EM examination of the seminiferous tubules of group III (vitamin B12 treated group) showed the same appearance of the Sertoli cells and germinal elements as in the control group. No marked changes had been noticed in the cells of this group (Fig. 4E).
In group IV, EM examination of the seminiferous tubules revealed cellular alteration of cells than those in the control groups. The Sertoli cells showed less extensive changes. Their nuclei kept homogenesity. The cytoplasmic organelles appeared less affected (Fig. 4F). Spermatogonia and primary spermatocytes are less affected, and the cytoplasm of both cells is more or less electron dense, the mitochondria are swollen and vacuolated, the chromatin particles clump periphery at nuclear membrane, the endoplasmic reticulum became vascular rather than tubular, and the basement membrane of the seminiferous tubules were more thickened with fibrous connective tissue than in control groups (Fig. 4G).
In the present study, 50 mg/kg of cimetidine in a daily dose for 52 days (group II) induced tubular cellular changes in the form of reduction in the thickness of the germinal epithelium, widening of the tubular cavities and apoptosis of the spermatocytes. The basement membrane was irregular with widened interstitial spaces containing congested blood vessels. Areas of loss of germ cells were also observed. Some detached germ cells were filling the tubular lumen. These changes were confirmed by the morphometrical analysis, which showed a significant increase in the shortest diameter of the tubules with significant decrease in the tubular luminal area and the epithelial areas. On the other hand, the luminal area was not significantly increased. The net result was alteration of the tubular shape and outline. Same results were reported by many authors [341819]. Beltrame et al. [3], reported a non-significant reduction in the luminal area and they explained this by the shrinkage of the tubular wall after loss of the germ cells. The Sertoli cells nuclei of the seminiferous tubules of this group were irregular in shape with their nuclei displaced away from the basement membrane. Previous studies reported a reduction in the number of these cells together with these changes [1920]. Studies proofed that drugs affecting germ cells without affection of the Sertoli cells produced an increase in the tubular lumen with maintained tubular outline [319], while drugs affect both Sertoli cells and germ cells produced irregular tubular outline [21]. This was explained by the fact that Sertoli cells are attached to the basement membrane by hemidesmosomes and the plasma membrane of the neighboring Sertoli cells by tight junctions and to the germ cells by several molecules that maintain Sertoli cell germ cell interaction [22]. Our study showed change in the tubular outline with increased tubular luminal area because cimetidine affects both Sertoli cells and germ cells.
Ultrastructural examination of testicular tissue of cimetidine-treated rats showed apparent changes in the Sertoli cells affecting mitochondria and other cellular organelles. These structural changes reflect functional abnormalities of the cells [23]. Sertoli cells were responsible for control of the normal functions of the testis and supported all stages of spermatogenesis [23]. Sertoli cells produce lactate from glucose under the effect of FSH. Any change in Sertoli cells function is expected to affect all functions of the testis. Sertoli cells functions are also known to be affected by the germ cells, particularly the spermatids through essential mediators that mediate gene expression and secretory activities of the cells [24]. The evident structural changes in the spermatids structure is also expected to affect the Sertoli cells function.
Our study revealed an increase in the thickness of the tubular basement membrane of cimetidine-treated rats; increased collagen contents explained this in the tubular wall caused by increased activity of the peritubular fibroblasts with subsequent fibrosis [25].
The testicular section of the rats treated with vitamin B12 (group III) alone showed almost no abnormality in both light microscopic and ultrastructural levels. Morphometrical measurements and ultrastructural examinations of this group showed nearly the same measurements of the controls.
The seminiferous tubules of the rats treated with cimetidine and supplemented with vitamin B12 (group IV) showed normal epithelial histoarchitecture and both tubular and epithelial areas were similar to those of the control group, and vitamin B12 treated group (groups I and III). However, some intercellular vacuolations with widened interstitium and some sloughed germ cells were seen in the tubular lumen.
The morphometrical evaluation of the group showed evident significant widening of the seminiferous tubules with increased tubular and epithelial areas compared with the control group. The luminal area was also significantly increased compared to the controls but decreased than the cimetidine-treated group. The basement membrane showed still apparent thickening. Ultrastructural examination of this group revealed mild changes in the Sertoli cells. Their nuclei appeared homogenous with vacuolated cytoplasm and almost normal cellular organelles. Some lipid droplets were seen in the cytoplasm.
Vacuolations suggest possible phagocytosis which is known event usually affects apoptosis [20]. These changes can be explained by healing of the testicular tissue, which is not yet complete, maybe because of the need for longer duration. The increased lipid contents of the cells can be explained by a reduction in steroid biosynthesis caused by a decrease in level of testicular steroids. Similar condition followed diabetes induction in experimental animals [23].
Our study revealed immune staining of the ERβ receptors in all layers of the germinal epithelium of the control rats. The cellular ERβ immunoreactivity was observed in spermatogonia, primary spermatocytes, and spermatid. Same findings were reported [41526]. Higher magnification examination detected the immune staining in the cytoplasm of the target cells. ERβ receptors are nuclear, yet its localization in the cytoplasm has been demonstrated [27], also studies reported its presence in the mitochondria [2829]. Other studies reported the controversial presence of these receptors [4]. However, in accordance with our study, the cytoplasmic presence of these receptors are more frequent [430].
A stronger immunostaining was detected in the germinal epithelium of the damaged tubules of the cimetidine-treated group (group II) with stronger cytoplasmic immune staining in the spermatogonia, primary spermatocytes and spermatids, the detached cells and the apoptotic cells. A possible explanation of the increased immunostaining in this group is increased affinity of the estrogen receptors (ERβ) in the cells due to a decrease in the intertubular hormone level caused by cimetidine.
The presence of the receptors in the round and elongated spermatids is related to the normal development of it [31]. This finding confirmed the important role of estrogen in maturation of the spermatids. The degree of immunoexpression of the cells was proofed to be caused by the variable degrees of affinities of the antibodies by the amino acid sequence [32]. The presence of immunoreactivity in the flagella and the residual bodies had also been reported [15]. This fact was explained by the presence of the aromatase enzyme; the enzyme which converts testosterone to estrogen, in the flagella and residual bodies.
Studies on estrogen receptors indicated a role of this hormone in down-regulation of the estrogen receptors [33]. Estrogen has a feedback control over FSH. The increased FSH, impaired Sertoli cells function and the possible intratubular estrogen deficiency caused by cimetidine can explain the increased presentation of ERβ indicated by increased immunoexpression in cimetidine-treated animals (groups II and IV). Immunoreactivity of the testicular sections of rats treated with vitamin B12 (group III) rats was very close to that of the control group.
ER immunostaining in testicular sections of treated rats with cimetidine and vitamin B12 (group IV) showed Sertoli cell vacuolations with variable degrees of cellular detachment in the tubular lumen. The detached cells showed positive immunoreactivity. The spermatids and primary spermatocytes' cytoplasm and nuclei were also immunopositive. Apoptotic cells with positive immunoreactions were less presented. The seminiferous tubules with maximal degree of destruction had strong immunoreactivity in the basal portion of their lining germ cells. The co-presence of vacuolated germ cells with ERβ positive cells suggest phagocytosis [19]. A proposed role of ERβ in the process of germ cell apoptosis had been postulated [19].
In the present study, it has been demonstrated that Sertoli cells are structurally affected by cimetidine, leading to Sertoli cell death and significant reduction in its number. It is known that Sertoli cells and germ cells can convert testosterone into estrogen via aromatase enzyme [34]. This conversion of testosterone into estrogen by aromatase enzyme in the damaged tubules by cimetidine is probably affected by Sertoli cell death. The co-presence of ERβ with aromatase in the same portion of the spermatids support the fact that spermiogenesis is an estrogen-dependent process [4].
A suggested interference possibility of cimetidine in androgenization was reported [35]. It was also demonstrated to have a toxic effect on the peritubular tissue [36]. Cimetidine was even proofed to affect the Sertoli cells-basement membrane interface [19]. It also caused reduction in the number of these cells due to cell death by apoptosis [19]. Sertoli cells and the germ cells convert testosterone into estrogen by aromatase enzyme [37]. The effect cimetidine on these cells adds to its antiandrogenic effect.
Vitamin B12 is essential for the maintenance of the testicular function [3]. Independently of the mechanism of action of cimetidine on the seminiferous tubules, vitamin B12 supplementation exerts beneficial effect on the cells stimulating spermatogenesis. With cimetidine treatment, this protective effect is more evident as evidenced by histopathological, ultrastructural and immunostaining studies.
The present study illustrated the histopathological and the ultra-structural changes mediated by cimetidine on the testicular tissues and on spermatogenesis. These changes are caused by the interference of cimetidine with the hormonal control of spermatogenesis as evidenced by affecting the ERβ receptors. The protective effect of vitamin B12 was reported.
Figures and Tables
Fig. 1
Seminiferous tubular shortest diameter, total tubular area, area of the seminiferous epithelium and luminal area: series 1 is a seminiferous tubular shortest diameter, series 2 is the total tubular area, series 3 is the area of the seminiferous epithelium, and series 4 is the luminal area

Fig. 2
Photomicrographs of sections of adult rat testis stained by hematoxylin and eosin demonstrating. (A) The control group showing normal seminiferous tubules and interstitial tissue and Leydig cells. The tubular germinal epithelium is formed of different types of spermatogenic cells; spermatogonia, primary spermatocytes, spermatids and sperms (×100). L, Leydig cells; SG, spermatogonia; SC, primary spermatocytes; ST, spermatids, sperms; Sz, spermatids. (B) The control group showing the normal tubular germinal epithelium formed of different types of spermatogenic cells: spermatogonia, primary spermatocytes, and spermatids, sperms (×400). (C) The cimetidine-treated group showing the seminiferous tubular epithelium (spermatogonia, primary spermatocytes, and spermatids) and interstitial tissue and Leydig cells. Marked atrophy of the seminiferous tubules with epithelial disorganization and reduced thickness of germinal epithelium is noted (×100). (D) Higher magnification of panel (C), the seminiferous tubules are damaged with darkly stained pyknotic nuclei (arrowheads). Many vacuoles of variable size are noticed in the germinal epithelium (arrows). Irregular basement membrane with wide interstitial spaces containing (×400). (E) The cimetidine and vitamin B12–treated group showing the seminiferous tubules, the interstitial tissue and Leydig cells. The germinal epithelium (spermatogonia, primary spermatocytes, and spermatids, sperms) is organized in concentric layers (×100). Scale bars=50 µm (A, C, E), 25 µm (B, D).
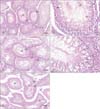
Fig. 3
Photomicrographs of sections stained with immunohistochemistry for detection of estrogen receptor β demonstrating. (A) The uterus (positive control) showing strong immunolabeling in the epithelium and endometrial glands (arrowheads). The connective and muscle tissue showed weak and moderate immunopositivity, respectively (×100). (B) Higher magnification of panel (A) (×400). (C) The testis of control rat showing evident cytoplasmic immunostaining for estrogen receptors in all layers of the germinal epithelium (arrowheads) including spermatogonia, primary spermatocytes, and spermatids (×400). (D) The testis of cimetidine-treated rat showing strong immunopositivity in both nuclei and cytoplasm of spermatids (arrowheads), in the cytoplasm of primary spermatocytes and in the germ cell of the basal layer (×400). P, primary spermatocytes; S, basal layer. (E) The testis of cimetidine-treated rat showing strong immunopositivity in both nuclei and cytoplasm of spermatids (arrowhead), in the cytoplasm of primary spermatocytes and in the germ cell of the basal layer (×400). Scale bars=50 µm (A), 25 µm (B–E).
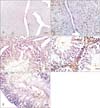
Fig. 4
Photoelectron micrographs of the rat testis demonstrating. (A) A control rat, showing the basement tubular membrane of the seminiferous tubules and myoid cells, primary spermatogonium, Sertoli cell cytoplasm with a large nucleus, homogenous nucleoplasm and prominent nucleolus. Its cytoplasm contains mitochondria. (B) A control rat showing Sertoli cell and spermatogonium with large round nucleus. (C) A treated rat testis with cimetidine, showing Sertoli cell resting on a basement membrane, with flat myoid cells. The cell has folded nuclear membrane with large cytoplasmic vacuole and lysosomes and swollen mitochondria with loss of the homogenicity of the nucleoplasm. (D) A treated rat testis with cimetidine, showing spermatogonia with an irregular nuclear membrane, cytoplasmic vacuoles, distorted mitochondria, and smooth endoplasmic reticulum. Note the fragments of the Sertoli cells in-between the germ cells with dark intercellular fragments and vacuolations (arrow). (E) Group III rat testis, showing Sertoli cell resting on the basement membrane with flat myoid cells. (F) Group IV showing the Sertoli cells resting on the tubular basement membrane with myoid cell. The Sertoli cell nucleus appears homogenous with vacuolated cytoplasm containing mitochondria, tubular endoplasmic reticulum, and lysosomes. (G) Group IV showing primary spermatocytes with a nucleus with clumped chromatin, dilated Golgi apparatus, mitochondria, and tubular endoplasmic reticulum. BM, basement tubular membrane; M, myoid cells; PS, primary spermatogonium; S, Sertoli cell; Mt, mitochondria; SP, spermatogonium; V, cytoplasmic vacuoles; L, lysosomes; ER, endoplasmic reticulum; ch, chromatin; GA, Golgi apparatus. Scale bars=5 µm (A–G).
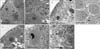
Table 1
Shortest diameter of seminiferous tubules, the total tubular area, the area of the seminiferous epithelium and the luminal area of the four groups

References
1. Kubecova M, Kolostova K, Pinterova D, Kacprzak G, Bobek V. Cimetidine: an anticancer drug? Eur J Pharm Sci. 2011; 42:439–444.

2. Kalra BS, Aggarwal S, Khurana N, Gupta U. Effect of cimetidine on hepatotoxicity induced by isoniazid-rifampicin combination in rabbits. Indian J Gastroenterol. 2007; 26:18–21.
3. Beltrame FL, Caneguim BH, Miraglia SM, Cerri PS, Sasso-Cerri E. Vitamin B12 supplement exerts a beneficial effect on the seminiferous epithelium of cimetidine-treated rats. Cells Tissues Organs. 2011; 193:184–194.
4. Sasso-Cerri E. Enhanced ERbeta immunoexpression and apoptosis in the germ cells of cimetidine-treated rats. Reprod Biol Endocrinol. 2009; 7:127.

5. Genissel C, Levallet J, Carreau S. Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells: comparison with estradiol production. J Endocrinol. 2001; 168:95–105.

6. McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003; 23:8701–8705.

7. Rago V, Maggiolini M, Vivacqua A, Palma A, Carpino A. Differential expression of estrogen receptors (ERalpha/ERbeta) in testis of mature and immature pigs. Anat Rec A Discov Mol Cell Evol Biol. 2004; 281:1234–1239.
8. Hamid Q, Hamid S, Minhas LA, Gul A. Influence of cimetidine and bromocriptine on prolactin levels in rat fertility. Int J Physiol Pathophysiol Pharmacol. 2009; 1:33–40.
9. Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003; 67:979–986.
10. Ruscin JM, Page RL 2nd, Valuck RJ. Vitamin B(12) deficiency associated with histamine(2)-receptor antagonists and a protonpump inhibitor. Ann Pharmacother. 2002; 36:812–816.

11. Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002; 129:1983–1993.

12. Yamada K, Kawata T, Wada M, Mori K, Tamai H, Tanaka N, Tadokoro T, Tobimatsu T, Toraya T, Maekawa A. Testicular injury to rats fed on soybean protein-based vitamin B12-deficient diet can be reduced by methionine supplementation. J Nutr Sci Vitaminol (Tokyo). 2007; 53:95–101.

13. Kawata T, Funada U, Wada M, Matsushita M, Sanai T, Yamada H, Kuwamori M, Arai K, Yamamoto Y, Tanaka N, Tadokoro T, Maekawa A. Breeding severely vitamin B12-deficient mice as model animals. Int J Vitam Nutr Res. 2004; 74:57–63.

14. Oshio S, Ozaki S, Ohkawa I, Tajima T, Kaneko S, Mohri H. Mecobalamin promotes mouse sperm maturation. Andrologia. 1989; 21:167–173.
15. Rago V, Siciliano L, Aquila S, Carpino A. Detection of estrogen receptors ER-alpha and ER-beta in human ejaculated immature spermatozoa with excess residual cytoplasm. Reprod Biol Endocrinol. 2006; 4:36.
16. Naraghi MA, Abolhasani F, Kashani I, Anarkooli IJ, Hemadi M, Azami A, Barbarestani M, Aitken RJ, Shokri S. The effects of swimming exercise and supraphysiological doses of nandrolone decanoate on the testis in adult male rats: a transmission electron microscope study. Folia Morphol (Warsz). 2010; 69:138–146.
17. Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol. 2001; 178:189–198.
18. Beltrame FL, Sasso-Cerri E. Vitamin B12-induced spermatogenesis recovery in cimetidine-treated rats: effect on the spermatogonia number and sperm concentration. Asian J Androl. 2017; 19:567–572.

19. Sasso-Cerri E, Miraglia SM. In situ demonstration of both TUNEL-labeled germ cell and Sertoli cell in the cimetidine-treated rats. Histol Histopathol. 2002; 17:411–417.
20. Sasso-Cerri E, Cerri PS. Morphological evidences indicate that the interference of cimetidine on the peritubular components is responsible for detachment and apoptosis of Sertoli cells. Reprod Biol Endocrinol. 2008; 6:18.

21. Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT. Intratesticular injection of adenoviral constructs results in Sertoli cell-specific gene expression and disruption of the seminiferous epithelium. Reproduction. 2009; 137:361–370.

22. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004; 25:747–806.

23. Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012; 15:623–635.
24. Yamamoto Y, Sofikitis N, Mio Y, Loutradis D, Kaponis A, Miyagawa I. Morphometric and cytogenetic characteristics of testicular germ cells and Sertoli cell secretory function in men with non-mosaic Klinefelter's syndrome. Hum Reprod. 2002; 17:886–896.

25. Hutson JC. Altered biochemical responses by rat Sertoli cells and peritubular cells cultured under simulated diabetic conditions. Diabetologia. 1984; 26:155–158.

26. Tirado OM, Selva DM, Toran N, Suarez-Quian CA, Jansen M, McDonnell DP, Reventos J, Munell F. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. J Androl. 2004; 25:84–94.
27. Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria: physiological and pathological implications. Mol Cell Endocrinol. 2008; 290:51–59.
28. Gruber HE, Yamaguchi D, Ingram J, Leslie K, Huang W, Miller TA, Hanley EN Jr. Expression and localization of estrogen receptor-beta in annulus cells of the human intervertebral disc and the mitogenic effect of 17-beta-estradiol in vitro. BMC Musculoskelet Disord. 2002; 3:4.

29. Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004; 101:4130–4135.
30. Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod. 2005; 20:3481–3487.
31. Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002; 87:2706–2715.
32. Selva DM, Tirado OM, Toran N, Suarez-Quian CA, Reventos J, Munell F. Estrogen receptor beta expression and apoptosis of spermatocytes of mice overexpressing a rat androgen-binding protein transgene. Biol Reprod. 2004; 71:1461–1468.
33. Agarwal VR, Sinton CM, Liang C, Fisher C, German DC, Simpson ER. Upregulation of estrogen receptors in the forebrain of aromatase knockout (ArKO) mice. Mol Cell Endocrinol. 2000; 162:9–16.

34. de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011; 9:93.

35. Sasso-Cerri E, Giovanoni M, Hayashi H, Miraglia SM. Morphological alterations and intratubular lipid inclusions as indicative of spermatogenic damage in cimetidine-treated rats. Arch Androl. 2001; 46:5–13.

36. Franca LR, Leal MC, Sasso-Cerri E, Vasconcelos A, Debeljuk L, Russell LD. Cimetidine (Tagamet) is a reproductive toxicant in male rats affecting peritubular cells. Biol Reprod. 2000; 63:1403–1412.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download