Abstract
Multiple strictures of small bowel induced by nonsteroidal anti-inflammatory drugs (NSAIDs), were known as diaphragm disease. The purpose of these case reports is to present 3 cases of diaphragm disease of small bowel and summarize the clinical features of this disease entity. A 34-year-old man, a 63-year-old man, and a 66-year-old woman were admitted to Daegu Catholic University Medical Center because of recurrent intestinal obstructions. Two of these patients had taken heavy NSAIDs use. Capsule endoscopy was performed in all cases and the all capsules were retained by circumferential strictures of the ileum. Segmental resection of the strictures was performed in 2 patients and 1 underwent just enterotomy and capsule removal. In conclusion, clinicians should be aware that diaphragm disease might be a cause of small bowel obstruction especially in patients receiving long term NSAIDs therapy.
Multiple strictures of the small bowel induced by nonsteroidal anti-inflammatory drugs (NSAIDs), also known as diaphragm disease, were first described by Lang et al. in 1988 [1]. Diaphragm disease is characterized by multiple, thin, concentric septa-like mucosal projections or broad circumferential ridges that narrow the small bowel lumen to a ‘pinhole.’ This can result in a subacute intestinal obstruction that sometimes requires laparotomy. Long-term use of NSAIDs is considered to be the most important causative factor of this disease entity, but several studies have reported cases with multiple diaphragm-like strictures in the small bowel that are not associated with the chronic use of NSAIDs [2]. With the widespread use of NSAIDs, an elderly society, and the advent of recent modalities such as capsule endoscopy (CE) and doubleballoon endoscopy (DBE), the incidence of diaphragm disease is on the rise and seems likely to increase in the future [34].
We report 3 cases of diaphragm disease requiring surgery from May 2012 to July 2016. We reviewed the clinical features of the disease by radiologic, endoscopic, operative, and pathologic findings.
The Institutional Review Board of Daegu Catholic University Medical Center is waived for case report.
A 34-year-old male was referred for evaluation of anemia from a local clinic. He had stool occult blood with hypochromic microcytic anemia (hemoglobin = 9 g/dL). At the local clinic, the source of bleeding could not be identified by esophagogastroduodenoscopy (EGD) or colonoscopy. He experienced intermittent abdominal pain and a 10-kg weight loss. The patient had no previous medical history and denied a history of NSAID ingestion. The vital signs were stable, and abdominal examination showed nonspecific findings without tenderness or distension.
For the investigation of small bowel bleeding, he had undergone CE, but the capsule was retained. Abdominal CT scan showed a retained capsule in the ileum without bowel distension or wall thickening. The capsule was removed successfully by DBE. CE and DBE revealed multiple ulcers and strictures in the ileum (Fig. 1A). We performed laparoscopic exploration and found diaphragm disease and five strictures within the ileum. Segmental resection of the ileum including the strictures was performed. On the microscopic examination, zonal fibrosis of submucosal tissues with focal mucosal erosion and mild chronic inflammation was observed (Fig. 1B); and the redundant submucosal tissue proximity to zonal fibrosis (circle) was compatible with septa-like structure. Epithelia were regenerated, but not evident for architectural distorsion or enhanced inflammation of crypts. The postoperative course was uneventful and has remained asymptomatic without recurrence.
A 66-year-old female presented with melena, intermittent abdominal pain, and vomiting for a month. She had a history of stroke, diabetic nephropathy, hypertension, and permanent pacemaker status due to bradycardia. Her past history also included a lumbar compression fracture for which she had been taking meloxicam, celecoxib, and combination drugs (acetaminophen and tramadol) for a long period. The physical examination showed mild abdominal distension with periumbilical tenderness, but the patient was afebrile and hemodynamically stable. Routine blood tests showed severe anemia (hemoglobin = 4.2 g/dL). EGD and colonoscopy were performed for evaluation of anemia, but the results were normal.
CE was undertaken and showed multiple circumferential ulcers surrounded by erythematous mucosa and short segmental strictures from the jejunum to the ileum (Fig. 2). However, the capsule was retained, and the patient presented with recurrent episodes of subacute small bowel obstruction. Abdominal CT scan showed the retained capsule with mild small bowel distension but no definite transitional zone. An attempt was made to perform endoscopic removal by DBE but was unsuccessful because the endoscopy did not reach the location of the capsule. At urgent laparotomy, multiple strictures within the ileum were found, but small bowel segmental resection was virtually impossible because the diseased segments were widespread. Stricturoplasty and capsule removal was performed by the reason of acceptable patency of the bowel lumen. Following immediate postoperative periods, the patient was able to take liquid diet and supplementary feeds, and remained well and asymptomatic without obstructive symptoms at 1-year follow-up.
A 63-year-old male presented with postprandial abdominal pain for 2 months. He had normal defecation but complained of borborygmi after meals. Past history included angina and migraine for which he had been taking a combination of drugs (acetaminophen and isopropylantipyrine) for about 30 years. The vital signs were stable, and the abdominal examination showed nonspecific findings without tenderness or distension. Abdominal CT scan revealed dilatation of a short segment of small bowel with a transitional segment in the lower abdomen, probably the middle or distal segment of the ileum (Fig. 3A). In spite of conservative management for a month, obstructive symptoms were not improved, and we decided to perform a laparoscopic exploration. Under laparoscopic view, the small bowel appeared to be normal without mild distension, and we extended the incision for palpation. Through mini-laparotomy, there were multiple short, annular, fibrotic strictures within a relatively wide range of ileum (Fig. 3B). Segmental resection of about 30 cm in length was performed on the ileum containing severe strictures. Microscopically, there existed multiple shallow ulcers with adjacent submucosal fibrosis and a variable degree of chronic inflammation (Fig. 3C, D). The regenerative surrounding epithelia were unremarkable. The postoperative course was uneventful.
At 3 months after surgery, the patient presented with melena and anemia (hemoglobin = 4.7 g/dL). Because the results of an EGD and colonoscopy were normal, CE was performed. The capsule was retained and showed multiple circular stenoses with erosions. Obstructive symptoms such as abdominal bloating, pain, and distension persisted. Finally, he underwent a reoperation and additional resection of stenotic segments. He was asymptomatic at 6-month follow-up.
Diaphragm disease is now considered to be an enteropathy secondary to NSAID-induced injury. NSAIDs are the most commonly prescribed drugs in the world, and while their adverse effects on the stomach and the duodenum are widely recognized, their effects on the small bowel are underreported. Although 50%–70% of patients on long-term NSAIDs develop certain forms of small bowel enteropathy, the incidence of NSAID-induced diaphragm disease is only around 2% [5]. This underreporting is probably due to their subclinical courses. Furthermore, most clinicians are unaware of the fact that patients taking NSAIDs can develop these strictures.
The exact pathogenesis of diaphragm disease remains poorly understood. However, damage induced by NSAIDs has been discussed extensively [14678]. Therefore, it is thought that a reduction in the mucosal synthesis of prostaglandins induced by NSAIDs results in damage to enterocytes and villous microcirculation, and disrupts mucosal integrity. The subsequent inflammatory reaction leads to circumferential ulceration that may result in mucosa vulnerable to bacteria or toxin. In the healing phase, submucosal granulation tissue matures into collagenous scar tissue, and these rings of scar tissue contract, like drawstrings across the bowel lumen, eventually forming diaphragm-like strictures. The microscopic feature of diaphragm disease is not pathognomonic, but usually shows multiple shallow ulcers, submucosal fibrosis and/or a variable degree of accompanying stromal inflammation, and less evident for the chronicity of crypt inflammation related to the inflammatory bowel disease or connective tissue disorder. Of note, it appears that NSAIDs produce these diaphragm-like strictures only in a few susceptible patients. Recently, it has been found that a specific cytochrome P450 2C9 polymorphism, which causes a decreased breakdown of NSAIDs, was significantly associated with an increased risk of diaphragm disease [9].
The relative risks of the different NSAIDs are not very clear, and many studies have shown that selective COX-2 inhibitors may be significantly less injurious to gastrointestinal (GI) tract than traditional NSAIDs [9]. Dosage is one factor affecting the plasma concentration of NSAIDs. In general, diaphragm disease has been associated with high doses taken daily [1]. Diaphragm disease can occur anywhere along the whole GI tract. However, the majority of instances are located in the small intestine. Many reports state that small bowel diaphragm disease is located predominantly in the ileum [28]. This may be because of differences in the bacterial flora and immune system between the jejunum and ileum. Colonic diaphragm disease has more recently been reported and most often has been related to slow-releasing or enteric-coated NSAID forms [78]. Because these NSAIDs have a longer half-life, they are more likely to reach the colon before they are entirely digested.
Definite diagnosis is challenging because symptoms are often nonspecific, and conventional radiological techniques are inaccurate. Therefore, it is important for the physician who is aware of the disease to consider the possibility of diaphragm disease. Laboratory tests may reveal microcytic anemia and hypoalbuminemia [4]. Plain abdominal radiographs are usually unhelpful. Barium studies may show the diaphragms, but they are as easily overlooked as thin-walled diaphragms resembling exaggerated plicae circulares [24]. A CT scan may show a degree of obstruction but is unable to identify the thin diaphragms. The advent of CE and DBE may facilitate evaluation of the small bowel. Although the diagnosis of small bowel diaphragm disease has improved with the introduction of CE, there is a risk of capsule retention if strictures are present [238]. In all our cases, CE was performed, and all the capsules were retained by circumferential strictures. Under these circumstances, it usually requires an exploratory laparotomy or DBE to remove the retained capsule, so it should be used cautiously. If an obstruction is suspected, DBE might be a better option; this also has the advantage of allowing biopsies for histology. DBE is a valuable and minimally invasive technique for the detection of diaphragm disease, and endoscopic treatment is possible [10]. Despite these advances, a correct preoperative diagnosis is made at laparotomy in most cases.
At laparotomy, the meticulous palpation of the suspicious lesions is essential for making the diagnosis. The lesions may be missed unless they are specifically sought because they affect only the mucosa and submucosal layers leaving an intact serosa, and the diaphragm may feel slightly thickened. Therefore, the role of laparoscopy appears to be limited because the bowel usually has a normal appearance [38]. In our case, we failed to identify diaphragm disease by laparoscopy. Through mini-laparotomy incision, we can decide on the extent of the diseased segment with palpation. Additionally, it is important to examine the whole intestine carefully because there is potential for the existence of concomitant strictures in other regions. If the diagnosis is uncertain, intraoperative enteroscopy may be used to explore and assess the extent of the lesion. The management of diaphragm disease in most reported cases entails segmental resection of the involved intestine because multiple lesions are located close together along the intestine [8]. Although not common, stricturoplasty may be an alternative choice for scattered lesions that are impossible to resect. Recently, with the development of DBE, endoscopic balloon dilation could be considered as an alternative option for diaphragm disease [10]. The risk of intestinal perforation with endoscopic balloon dilation would be low because of the fibrotic features of the diaphragm disease.
The prognosis would be good if the offending NSAIDs could safely be withdrawn. The benefit of continuing with NSAIDs may outweigh the risk of GI injury for some patients; therefore, this needs to be considered on a case-by-case basis [3]. The use of prostaglandin derivatives (such as misoprostol) may protect against NSAID-induced GI damage; therefore, their concomitant use should be considered in patients who are particularly at risk of NSAID-associated GI complications where NSAIDs cannot be withdrawn [4]. A previous study has reported that a recurrence rate as high as 50% after surgery may be caused by the surgeon's failure to appreciate the extent of the lesions at the initial operation or a true recurrence due to continued use of NSAIDs [1].
In conclusion, the diagnosis of diaphragm disease is possible with a thorough understanding and careful interpretation of the disease. Especially, clinicians should be aware that diaphragm disease might be a cause of small bowel obstruction in patients receiving NSAIDs. It is important to confirm the diagnosis by meticulous palpation because the outer appearance shows only subtle changes. Further studies are needed to evaluate the pathogenesis, the risk factors, newer diagnostic methods, and treatment including medication.
Figures and Tables
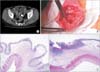
Fig. 1
(A) Double-balloon colonoscopy shows a diaphragm-like stricture in the ileum and the retained capsule. (B) The representative microscopic feature is submucosal fibrosis with insignificant inflammation and redundant submucosal tissues (circle) of septa-like structure (H&E, ×20).

Fig. 3
(A) Abdominal CT scan showed thickening of bowel wall (white arrow) in the middle or distal segment of the ileum. (B) Longitudinal section of small bowel revealed multiple circumferential strictures with a pinhole opening. (C, D) The histologic evaluation shows multiple shallow ulcers with zonal fibrosis and chronic inflammation of submucosal connective tissues (H&E, ×20).
References
1. Lang J, Price AB, Levi AJ, Burke M, Gumpel JM, Bjarnason I. Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal antiinflammatory drugs. J Clin Pathol. 1988; 41:516–526.

2. Wang ML, Miao F, Tang YH, Zhao XS, Zhong J, Yuan F. Special diaphragm-like strictures of small bowel unrelated to non-steroidal anti-inflammatory drugs. World J Gastroenterol. 2011; 17:3596–3604.

3. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011; 13:463–466.

4. Wang YZ, Sun G, Cai FC, Yang YS. Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal antiinflammatory drugs. Gastroenterol Res Pract. 2016; 2016:3679741.

5. Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007; 5:1040–1045.

6. Zhao B, Sanati S, Eltorky M. Diaphragm disease: complete small bowel obstruction after long-term nonsteroidal anti-inflammatory drugs use. Ann Diagn Pathol. 2005; 9:169–173.

7. Onwudike M, Sundaresan M, Melville D, Wood JJ. Diaphragm disease of the small bowel--a case report and literature review. Dig Surg. 2002; 19:410–413.
8. Slesser AA, Wharton R, Smith GV, Buchanan GN. Systematic review of small bowel diaphragm disease requiring surgery. Colorectal Dis. 2012; 14:804–813.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download