Abstract
Purpose
To compare survival outcomes between bile duct segmental resection (BDR) and pancreatoduodenectomy (PD) for the treatment of middle and distal bile duct cancer.
Methods
From 1997 to 2013, a total of 96 patients who underwent curative intent surgery for middle and distal bile duct cancer were identified. The patients were divided into 2 groups based on the type of operation; 20 patients were included in the BDR group and 76 patients were in the PD group. We retrospectively reviewed the clinical outcomes.
Results
The number of lymph nodes (LNs) was significantly greater in patients within the PD group compared to the BDR group. The total number of LNs was 6.5 ± 8.2 vs. 11.2 ± 8.2 (P = 0.017) and the number of metastatic LNs was 0.4 ± 0.9 vs. 1.0 ± 1.5 (P = 0.021), respectively. After a median follow-up period of 24 months (range, 4–169 months), the recurrence-free survival of the PD group was superior to that of the BDR group (P = 0.035). In the patients with LN metastases, the patients undergoing PD had significantly better survival than the BDR group (P < 0.001).
Neoplasm of the biliary tract can be divided into intrahepatic (6%) and extrahepatic (94%) according to location. In the case of intrahepatic cholangiocarcinoma, hepatic resection is inevitable. However, the surgical treatment for extrahepatic cholangiocarcinoma is dependent on the location of the tumor. Extrahepatic bile ducts are divided into hilar (49%), middle (25%), distal (19%), and diffused type (7%) and there are corresponding surgical treatment modalities [123]. For the treatment of mid to distal bile duct cancer, pancreatoduodenectomy (PD) is the procedure of choice. However, bile duct segmental resection (BDR) is now often attempted for middle common bile duct (CBD) cancer [456].
PD is associated with high morbidity and mortality. There are life threatening complications associated with PD that include severe pancreatic fistula and postpancreatectomy hemorrhage [78]. Postoperative pulmonary complications are also an important factor in the mortality of elderly patients undergoing PD [79]. In addition, complications relating to quality of life for patients, include postoperative diabetes mellitus [1011].
BDR and regional lymph node dissection (LND) might be an option, if the tumor is confined to the middle CBD. Surgeons can avertible p ancreatic fistula by BDR.
There are few published studies on the survival outcomes of BDR for middle CBD cancer compared to PD.
On the other hand, PD is superior to BDR in the aspect of radical surgery. However, the oncologic outcome has not been comprehensively compared with PD. The studies that have been done have had small patient numbers and therefore the evidence is slim.
The aim of this study was to analyze the survival outcomes after BDR for middle and distal CBD cancer and compare them to PD. In doing so we sought evidence for whether BDR should be considered as an alternative treatment modality. Moreover, LN metastasis and resection margin status, are known to be prognostic factors associated with extrahepatic bile duct cancer after surgical resection [1213]. Therefore, we validated known prognostic factors and investigated other risk factors.
A total of 120 patients who underwent surgery for middle and distal CBD cancer between 1997 and 2013, were investigated retrospectively. All patients were confirmed with CBD cancer in final pathology results and did not include ampullar of vater cancer. Cases which had tumor extension to the hilar bile duct bifurcation were not included in this study. Twenty four patients who underwent palliative surgery, had distant LN metastasis, or had double primary cancers were also excluded. We analyzed the clinical data of 96 patients who underwent curative intent surgery for middle and distal CBD cancer. These patients were divided into 2 groups according to the type of operation; 20 patients had BDR and 76 patients PD. The PD group included patients who underwent pylorus preserving PD and conventional Whipple's procedure.
For BDR, a surgeon performed Kocher maneuver to elevate the pancreas head, followed by dissection of the CBD from the head of the pancreas. Proximal and distal bile duct was transected and the margin was routinely checked during the operation with frozen sections. The pathologist confirmed that resection margins were free of tumor. However, if there was tumor invasion to the distal resection margin of bile duct, the intrapancreatic bile duct was additionally removed. If the lowest bile duct margin was tumor positive, it was converted to PD. Proximal bile duct margin was evaluated in the same manner. If there was tumor invasion to the proximal bile duct margin, the hilar bile duct was also removed. Hepatic resection was strictly considered according to the resection margin case by case. However, patients who underwent hepatectomy were not included in this study.
The extent of LND was similar between BDR and PD group. In BDR group, the common hepatic, hepatoduodenal, and retropancreatic LNs were removed. The celiac or superior mesenteric LNs were not routinely removed. If there were grossly enlarged LNs or appeared suspicious on preoperative images, they were removed.
We retrospectively investigated age, sex, type of surgery, tumor location, tumor size, total number of dissected LNs, number of pathologically cancer cell positive LNs, R status, gross type, cell differentiations, T and N stage. The T and N stages were based on American Joint Committee on Cancer (AJCC) 7th edition. We also investigated whether adjuvant chemotherapy or adjuvant radiation therapy was performed after surgery. Factors relating to postoperative outcomes were hospital mortality, overall survival, disease-free survival, and tumor recurrence pattern.
Categorical variables are represented as numbers (percentages). Continuous variables are shown as median (range). The difference between groups was assessed by univariate analysis using the chi-square and Fisher exact test. Cox regression test was used for statistical significance in multivariate analysis of significant factors in univariate analysis. Kaplan-Meier survival analysis was used to analyze overall survival and disease free survival between the 2 groups. P-values less than 0.05 were considered statistically significant.
A total of 96 extrahepatic cholangiocarcinoma patients were included and analyzed according to the inclusion criteria; twenty patients in the BDR group and 76 patients in the PD group.
In the PD group, there were five converted cases from BDR that had a positive distal bile duct resection margin in the frozen section during operation. Seven patients of 20 in the BDR group (35%) showed R1 resection, and 3 of them were R0 in frozen biopsy during surgery but tumor invasion was confirmed in permanent pathology. In the PD group, 5 patients (6.6%) had positive resection margin on frozen and permanent pathology. Two of the 5 patients with margin positive were radial margin positive state and had no adjacent organ invasion. Therefore, additional resection was not performed. The other 3 patients showed proximal margin positive results. One of the 3 patients showed moderate dysplasia and the other 2 showed invasive adenocarcinoma. High hilar resection was performed as much as possible when the proximal resection margin was positive. There was no further resection when the proximal margin positive status was repeated and no visible cancer. Two of these patients had reccurence at the choledochojejunostomy site.
In-hospital mortality was observed in 1 patient (5%) with BDR and 6 patients (7.9%) with PD. There were no deaths from surgical site complications such as pancreatic fistula or postpancreatectomy hemorrhage. Six mortality cases in the BDR and PD groups were all pulmonary complications from postoperative pneumonia with one exception. One patient expired from organ space site infection without pancreatic fistula. The patient who died after BDR was 76 years old. Four of the patients who died after PD were aged from 74 to 94. These patients were older and had difficult lung care after surgery. One patient was 69 years old, but asthma was the underlying cause and the risk of pulmonary complications was high (Fig. 1).
The median age of patients was 69 years (range, 34–88 years) and the median follow-up period was 23.5 months (range, 1–170 months).
There were no statistically significant differences between BDR and PD groups in age, sex, tumor size, gross type, histologic grade, adjuvant chemotherapy, and adjuvant radiation therapy.
However, the tumor location, T stage, total number of LNs dissected, the number of positive LNs and R status were significantly different between the 2 groups.
In cases of middle CBD cancer, BDR was the primary surgery. Among middle CBD cancer patients, 20 patients (80%) had BDR and 5 patients (7%) had PD. When the tumor involved distal CBD 4 patients (20%) had BDR and 71 patients (93%) had PD. These results showed statistically significant differences. (P < 0.001).
The T stage was also different between the 2 groups. In the BDR group, 13 patients (65%) had T2 stage and 41 patients (54%) had T3 stage (P = 0.003).
The number of LNs was significantly higher in the PD group than in the BDR group. The total number of LNs was 6.5 ± 8.2 in BDR group versus 11.2 ± 8.2 (P = 0.017) in PD group and the number of metastatic LNs was 0.4 ± 0.9 versus 1.0 ± 1.5 (P = 0.021), respectively. R0 resection rate was lower in the BDR group. Thirteen patients (65%) had R0 resection margin compared with 71 patients (93%) in the PD group (P = 0.001) (Table 1).
In multivariate analysis, factors related to recurrence were operation type, resection margin, and LN metastasis. BDR was associated with a higher risk of cancer recurrence than PD (P = 0.045). R1 resection margin and LN metastases were statistically significant as a poor prognostic factors (P = 0.049 and P = 0.004, respectively) (Table 2).
Survival analysis included only data from 89 patients out of 96 patients; 6 patients with early mortality and 1 patient with hopeless discharge were excluded.
Of the median follow-up period (24 months; range, 4–169 months), overall survival was not significantly different, but the recurrence-free survival rate of the PD group was superior to that of the BDR group (P = 0.035). There was no significant difference in recurrence-free survival between BDR and PD patients in the group without LN metastasis. But, for patients with LN metastasis, the survival rate of patients without recurrence was significantly higher in PD group than in BDR group (P < 0.001). R status did not show a significant difference between BDR and PD in recurrence free survival (Fig. 2).
There were some differences in the cancer recurrence pattern between the BDR and PD groups according to the LN metastatic stage. There were no LN metastases (N0) in 16 of 20 patients in the BDR group. Of the 20 patients in the BDR group, 16 (80%) had no LN metastasis (N0) and 4 (20%) had LN metastasis (N1). Of the 16 patients without LN metastasis (N0), 11 patients experienced recurrence; 6 were local regional or residual bile ducts recurrence, and 5 had distant metastases.
However, distant metastases were more common in patients with N1 in the BDR group. In the PD group, regardless of LN metastasis, distant organ recurrence was more common than local recurrence (Fig. 3).
Previous studies reported that the resection margin and the number of involved LNs were considered to be the strongest associations for middle and distal CBD cancer [14]. However, there remains controversy about prognostic factors. Most of those studies emphasized that LN involvement is more important in predicting prognosis than resection margin, but other studies reported that resection margin statistics are more important [15161718192021].
In our study, there were significant differences between the BDR group and the PD group in the location of tumor, T stage, number of resected LNs, and R status. The number of resected LNs was higher in PD than in BDR. There was no significant difference in overall survival between the 2 groups, but the PD group showed more favorable results in disease-free survival. In our data, PD in middle and distal CBD cancer patients with LN metastasis had a lower recurrence rate than BDR. R status was a prognostic factor related to recurrence, but there was no difference in recurrence-free survival rate between BDR and PD groups. Therefore, R0 resection is an important way by which to reduce the recurrence rate of both groups.
The number of LN in the PD group was higher than in the BDR group. This suggests that PD is a more advantageous method for dissection of peripancreatic LNs.
Until recently, the surgical treatment of choice for middle and distal CBD cancer has been PD, in order to obtain free surgical margins and in order to harvest the correct number of LNs for correct staging [9]. However, several recent studies report that BDR should be considered as a new surgical option for middle and distal CBD cancer [456]. Unfortunately, there is still little solid evidence to consider using BDR as an alternative to PD. In cases of LN enlargement, suspected LN metastasis at preoperative imaging, and positive distal resection margins at frozen biopsy during BDR, PD is the recommended procedure. BDR can be considered in more limited circumstances, if it is confirmed that the patient has a middle CBD cancer with no suspicion of LN metastasis in the early stages.
There were several limitations relevant to this study, including that it was based on a relatively small number of patients and that it was a retrospective study rather than a randomized controlled trial. In addition, it was difficult to analyze other parameters suggested as prognostic factors; for example perineural invasion and lymphovascular invasion that have previously been considered. As a consequence therefore, these results need to be validated by further well designed studies.
In conclusion, surgeons should carefully evaluate the patient's condition by carefully reviewing the various preoperative imaging tests before performing BDR for the middle and distal CBD cancer patients. If LN metastasis is suspected, PD is recommended as the surgical treatment of choice.
Figures and Tables
 | Fig. 2Kaplan-Meier survival curves between bile duct resection group and pancreatoduodenectomy group. (A) Disease free survival (n = 89). (B) Overall survival (n = 89). (C) No lymph node metastases (n = 89). (D) Lymph node metastases (n = 89). (E) R0 resection (n = 77). (F) R1 resection (n = 12). |
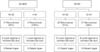 | Fig. 3The difference between BRD and PD group of recurrent rate and recurrent pattern according to N staging. BDR, bile duct segmental resection; PD, pancreatoduodenectomy. |
References
1. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463–473.
2. Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008; 15:41–54.
3. Skipworth JR, Keane MG, Pereira SP. Update on the management of cholangiocarcinoma. Dig Dis. 2014; 32:570–578.

4. Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998; 123:131–136.

5. Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007; 13:1505–1515.

6. Lee HG, Lee SH, Yoo DD, Paik KY, Heo JS, Choi SH, et al. Carcinoma of the middle bile duct: is bile duct segmental resection appropriate? World J Gastroenterol. 2009; 15:5966–5971.
7. Adam U, Makowiec F, Riediger H, Schareck WD, Benz S, Hopt UT. Risk factors for complications after pancreatic head resection. Am J Surg. 2004; 187:201–208.

8. Chang YR, Kang MJ, Kim H, Jang JY, Kim SW. The natural course of pancreatic fistula and fluid collection after distal pancreatectomy: is drain insertion needed? Ann Surg Treat Res. 2016; 91:247–253.

9. Sukharamwala P, Thoens J, Szuchmacher M, Smith J, DeVito P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB (Oxford). 2012; 14:649–657.
10. Kapoor VK. Complications of pancreatoduodenectomy. Rozhl Chir. 2016; 95:53–59.
11. Bock EA, Hurtuk MG, Shoup M, Aranha GV. Late complications after pancreaticoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2012; 16:914–919.

12. Todoroki T, Kawamoto T, Koike N, Fukao K, Shoda J, Takahashi H. Treatment strategy for patients with middle and lower third bile duct cancer. Br J Surg. 2001; 88:364–370.

13. Sasaki R, Takahashi M, Funato O, Nitta H, Murakami M, Kawamura H, et al. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery. 2001; 129:677–683.

14. Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005; 241:77–84.

15. Kiriyama M, Ebata T, Aoba T, Kaneoka Y, Arai T, Shimizu Y, et al. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015; 102:399–406.
16. Konishi M, Iwasaki M, Ochiai A, Hasebe T, Ojima H, Yanagisawa A. Clinical impact of intraoperative histological examination of the ductal resection margin in extrahepatic cholangiocarcinoma. Br J Surg. 2010; 97:1363–1368.

17. Bahra M, Jacob D, Langrehr JM, Neumann UP, Neuhaus P. Carcinoma of the distal and middle bile duct: surgical results, prognostic factors, and long-term follow-up. J Hepatobiliary Pancreat Surg. 2008; 15:501–507.

18. Hernandez J, Cowgill SM, Al-Saadi S, Villadolid D, Ross S, Kraemer E, et al. An aggressive approach to extrahepatic cholangiocarcinomas is warranted: margin status does not impact survival after resection. Ann Surg Oncol. 2008; 15:807–814.

19. Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007; 95:207–212.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download