Abstract
Cyanoacrylate closure, VenaSeal system, for the treatment of incompetent saphenous vein is a new technique. We report a successful case with a large great saphenous vein of 2.84 cm in diameter.
Treatment of incompetent saphenous veins has undergone wide changes during the past decade. Previously, surgical stripping was the primary choice of treatment. However, it has been largely replaced by endothermal ablation, either with radiofrequency or laser energy [1].
Recently, a new concept of treatment, cyanoacrylate closure (CAC), has been approved for the treatment of incompetent saphenous veins. The VenaSeal Closure System (Medtronic, Minneapolis, MN, USA), a new techniqueusing CAC, received the Conformité Européene (CE) mark in September 2011 and was approved by the U.S. Food and Drug Administration for closure of lower extremity superficial truncal veins in February 2015 [2]. In Korea, CAC for treatment of incompetent saphenous veins was approved in November 2016 as a new technology and announced by the Ministry of Health and Welfare in December 2016.
Several previous studies have demonstrated the safety and effectiveness of the VenaSeal system for the treatment of incompetent saphenous veins [34567].
However, all previous studies reported treatment of saphenous veins with diameters less than 2 cm.
We report a successful case with VenaSeal system for the treatment of recurrent varicose veins of 2.84 cm in diameter of great saphenous vein (GSV).
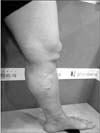
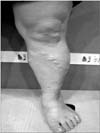
A 57-year-old female visited Charm Vein Center (Seoul, Korea) in April, 2017. She complained of intermittent night cramps, heaviness, itching, and swelling in her left leg. She had undergone surgical stripping 7 years prior on the left GSV. The clinical, etiologic, anatomic, pathophysiologic classification was C4a. She had eczema and pigmentation around the left ankle (Figs. 1, 2). A written informed consent was obtained.
Her initial score of the revised Venous Clinical Severity Scores (rVCSS) and Aberdeen Varicose Vein Questionnaires (AVVQ) were 7 and 23.278, respectively.
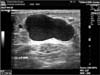
On a duplex sonogram, left sapheno-femoral junction (SFJ) was identified unclearly due to the previous surgery. Several neovascularizations were detected from the inguinal area and they gathered together as a new GSV and went caudally. The GSV was tortuous and reached a maximal diameter at the middle thigh level of 2.84 cm in diameter (Fig. 3).
With ultrasound guidance, a 5F. delivery catheter in 7F. introducer sheath was advanced to the inguinal area. Because a definite SFJ was indistinct due to the previous operation, the delivery catheter tip was positioned 5.0 cm distal to the proximal end of GSV. The proximal end of the saphenous vein was compressed thoroughly by the ultrasound probe with the left hand, at 2 cm proximal to the delivery catheter tip. Two injections of approximately 0.10-mL cyanoacrylate glue were each given 1 cm apart at this location, followed by a 3-minute period of local compression with the right hand. Then, repeated single injections and 30 seconds of compression for every 3 cm distally. Additional glue injection was allowed by the author's discretion for areas with large diameter, areas with communicating vein, or with a perforating vein.
At the point of the largest GSV with a diameter of 2.84 cm, to seal the large vein properly, we did double glue injections at each injection point for every 2 cm. This was performed 3 times, so the segment of 6-cm length GSV including the largest area at 2.84 cm was covered properly.
The remaining GSV was treated in the previously mentioned manner, with repeated single injections and 30-second compression for every 3 cm distally. The sequences were finished at the area of knee level.
Finally, the sheath/catheter was removed and compression was applied to the entry site until hemostasis was achieved. A small bandage was applied, and occlusion was confirmed by ultrasound.
No concomitant phlebectomy was done, but concomitant sclerotherapy with sodium tetradecyl sulfate was carried out after the above sequences were finished. Liquid type solution (0.2%) was used for the treatment of telangiectasia in the medial thigh area while foam type solution (1.0%) was used for the treatment of 2 perforating veins in the lateral calf and posterior calf area.
The patient was recommended to wear a thigh high compressive stocking for 1 week due to the concomitant sclerotherapy. Due to the patient's request for sedation, the procedure was performed under intravenous sedation in the presence of an anesthetist.
The total length of treated vein was 38 cm and the injection count was 20. Numerical Pain Rating Scale score was 1 at 6 hours after procedure, and remained at 0 at 1 day, 10 days, and 1 month after procedure.
Upon 1-month follow-up, we checked the duplex sonogram. The largest area of GSV was confirmed to be properly sealed. The maximal vein diameter was decreased from 2.84 cm to 2.31 cm. No blood flow was detected in the sealed area nor in the proximal area (Figs. 4, 5).
rVCSS at 1 month after procedure decreased to 2. AVVQ was not checked at 1 month. No other complications occurred such as infection, phlebitis, deep vein thrombosis or paresthesia.
This is the first report to describe the CAC, VenaSeal system, for the treatment of large recurrent varicose vein with a diameter of more than 2 cm.
One of good aspect of CAC is that it doesn't cause any mechanical or thermal damage with the additional glue injections.
Gibson et al. previously reported that in areas with a perforating vein, additional glue injection was allowed [6]. We have experienced more than 80 cases with the VenaSeal system, and we are also injecting additional glue in areas with large diameters or with a perforating vein. According to our previous experiences, this manner showed satisfactory outcomes.
However, CAC, VenaSeal system, is a new technique and a definite maneuver has not been established yet, especially for treatment of large tortuous or recurrent varicose veins.
More investigation and experiences should be carried out to support the treatment for large veins or recurrent varicose veins.
Figures and Tables
References
1. US markets for varicose vein treatment devices 2013: Medtech 360. Toronto (Canada): Millennium Research Group Inc.;2013.
2. U.S. Food and Drug Administration. Approval Order: Venaseal Closure System-P140018 [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2015. 02. 20. updated 2018 Feb 23. cited 2017 Mar 28. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=p140018.
3. Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology. 2015; 30:397–404.

4. Proebstle TM, Alm J, Dimitri S, Rasmussen L, Whiteley M, Lawson J, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015; 3:2–7.

5. Morrison N, Gibson K, McEnroe S, Goldman M, King T, Weiss R, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015; 61:985–994.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download