Abstract
Purpose
We introduce a training porcine model for laparoscopic common bile duct (CBD) repair with T-tube insertion. The model could be the feasible training tool for a surgeon learning hepatobiliary surgery.
Methods
Totally laparoscopic CBD repair with T-tube insertion was performed on 9 pigs by 9 trainees naïve in hepatobiliary surgery. Similar to the situation of iatrogenic injury, CBD was transected by laparoscopic scissors at the middle part about 1 cm in length, and the transected CBD was repaired through end-to-end anastomosis with T-tube insertion. A secureness of anastomosis was confirmed by saline leakage test and all animals were sacrificed after the surgery.
Since the 1990s, laparoscopic cholecystectomy (LC) has replaced the open cholecystectomy and become the gold standard procedure for symptomatic cholelithiasis or benign gallbladder (GB) disease such as GB polyp. However, common bile duct (CBD) injury during the operation still occurs in 0.5%–0.7% of LC procedures [1], even with experts who are specialized for hepatobiliary surgery. CBD injury can result in the fatal outcomes such as bile peritonitis, sepsis and death. Progression to the biliary stricture can result in recurrent cholangitis, obstructive jaundice, or biliary sepsis.
In many cases of CBD injury found intraoperatively, laparoscopic CBD repair accompanying with T-tube insertion is preferred as the definite management for this complication. The single-stage procedure does not require further intervention. However, proficiency of the operator for laparoscopic CBD manipulation is necessary.
For those who are novices at the technique, surgical training model for laparoscopic CBD manipulation would be advantageous. Practicing the specific procedure or operation should be required prior to the actual clinical situation [2]. A hands-on training model could be a very feasible and effective educational tool to allow surgeons to acquire the specific surgical technique or skills [34].
Here, we report the development and use of a porcine training model of laparoscopic CBD repair with T-tube insertion. The feasibility of the model as an effective training tool for trainees who are not expert in hepatobiliary surgery is demonstrated. We also added the several tricks and tact to conduct this technique more efficiently and simply.
All experimental operations were performed in the laparoscopic training center and the current study was approved by the institutional animal care and use committee of the hospital.
Three-month-old female pigs 31 to 35 kg in weight underwent bowel enema at midnight the day of surgery and were fasted for 24 hours before the surgery. The operative procedures were performed by general surgery residents. They had performed fewer than 15 cases of LC and had no experience with laparoscopic CBD manipulation. Prior to the operation, residents were educated about the whole process of the operation through a video review. Surgery was supervised by a surgeon specialized in hepato-biliary pancreas surgery with experience of over 100 cases of laparoscopic CBD manipulation. The operation time, intraoperative blood loss, and CBD diameter were recorded. To confirm the security of anastomosis for bile leakage at the anastomosis site, a saline solution of indocyanine green was flushed through T-tube. The whole operation time and the average time to perform the CBD repair with T-tube insertion using the laparoscopic suture were accurately calculated through the video decipher and operative records.
Under general anesthesia, 4 trocars were placed as the same manner in the standard LC [5]; 10-mm trocar at the umbilicus for the laparoscope and three 5-mm trocars were used for the retraction of GB, with the the surgeon's right and left hand for working channels, respectively (Fig. 1). After establishment of pneumoperitoneum with 12-mmHg carbon dioxide, the pig was placed in the reverse Trendelenburg position tilted to the left.
The operation was initiated by liver retraction through the direct suture to lift up the hepatic lobe adjoined to the GB and the hepatoduodenal ligament was dissected carefully to expose the CBD. The anatomical structure of porta hepatis in porcine has several characteristic features in common with those in humans [67]. The porcine cystic duct is buried in the adjacent hepatic parenchyme deeply that makes it hard to distinguish from the surroundings. The diameter of the porcine CBD is 2.3–3.0 mm, which is a bit smaller than that of humans. However, it runs to the right side of hepatic artery and lies in front of portal vein, as in humans [8]. This enables dissection of porcine CBD as is done in humans.
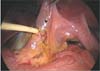
The Hartman's pouch of the GB was retracted superiorly and laterally by the first assistant using the laparoscopic grasper to facilitate the dissection. After the identification of anatomical structure around the CBD, the CBD was transected by laparoscopic scissors at the midpart and a segment of CBD about 1 cm in length was resected, similar to common cases of iatrogenic CBD ransection (Fig. 2).
The first step of CBD repair used 2 stay sutures without making the knot on both corners of the CBD resection line to sustain the tension of suture lines during the anastomosis. The stay sutures were taken out separately; the stay suture on the right side was taken out from the body through the lateral port, whereas the left one was laid in the peritoneal cavity with a laparoscopic clip on tips to help sustain the tensile force appropriately during the CBD repair by lifting it. After making the stay sutures, the posterior side of CBD incision was closed with 2 interrupted stitches using absorbable 5–0 multifilament sutures from to right to left side. A latex rubber 8F T-tube was inserted after cutting the T limb to an adequate size. The 2 short limbs were about 1.0 and 1.5 cm long, respectively; the longer limb was inserted toward the distal side of CBD and the shorter limb was introduced upwards for the prevention of the dislocation of tube. When the tube was positioned in place, the anterior side of CBD incision was closed from right to left using about 2 or 3 interrupted sutures snugly around the T-tube. After closure of CBD incision, stay sutures at both edge of anastomosis line were tied. The opposite end of T-tube was brought out through the minimal incision in the upper abdominal wall and the bile leakage test was performed (Fig. 3). If there was any evidence of leakage, such as the flow of greenish fluid from the anastomosis site, an additional suture was applied. After the establishment of anastomosis security, the cystic duct was divided and standard LC was performed. After operation, all animals were sacrificed.
Nine domestic pigs underwent the operation during the study period. Surgery was performed by nine junior residents who had not performed laparoscopic CBD exploration. The preoperative characteristics of the animals are provided in Table 1. Total mean operative time was 85 ± 1.7 minutes (range, 84–86 minutes). To estimate the consumed time in more detail, the mean time spent performing the CBD repair with T-tube insertion was calculated as 71 ± 3 minutes (range, 68–74 minutes). After deducting the time spent for T-tube manipulation, total anastomosis time for CBD repair was 67.5 ± 1.5 minutes (range, 66–69 minutes). The average number of knots needed to complete the anastomosis was 7.4 ± 0.2 (range, 6–8). Bile leakage after primary anastomosis occurred in 1 case (11.1%) which needed 1 additional suture on the anterior aspects after anastomosis. There was no significant intraoperative bleeding requiring fluid resuscitation or which caused instability of vital signs during the operation. All operators completed the task successfully. The surgical outcomes are also described in Table 1.
Iatrogenic CBD injury during LC still occurs despite of the remarkable progress in surgical techniques and instruments, and can involve expert and novice surgeon alike. Laparoscopic CBD repair with T-tube insertion is a useful solution to this complication, and can permit the intraoperative, single-stage management without additional intervention in the postoperative period. The procedure can be performed in cases with partial CBD resection or cases having segmental CBD resection that can be repaired through primary end-to-end repair without tension [910]. However, the surgical outcome is influenced by operator proficiency and skill. The approach is challenging for a novice surgeon. As a solution, a well-designed training animal model could be an effective tool for practice and mastery of the method. Gaining experience in the surgery and handling the necessary equipment could equip surgeons to better and more safely perform the operation in actual clinical situations.
Pigs have very similar characteristics with humans in terms of the internal organ or vascular structures [7811]. Porcine organs are relatively comparable in size or shape with humans than those of other mammals including the rat, dogs, and rabbit. These properties are beneficial for application as the surgical training model, and pigs are been widely used for this purpose, especially for laparoscopic procedures [12]. The living porcine model has the several advantages that an artificial material or nonliving model cannot meet. This living model maximally simulates the human situation because of ongoing respiration and pulsatile movements of vascular structures. The tactile sense of the manipulated tissue is almost the same as human tissue, which heightens the value of the simulation [1314]. These characteristics make the living animal model optimal for the evaluation of the safety or feasibility of a surgical technique [15].
However, despite of these advantages of living porcine model, there are a number of problems. The model is relatively costly, since specialized animal care by veterinarian or additional equipment can be needed. Therefore, limited medical resources can preclude repeated use of the porcine model.
With the accumulated experience through the training, authors have devised several tracks and tact which help to improve efficiency of training. The present data demonstrate the feasibility of the model and the satisfactory training results, especially regarding the clinical callowness of the operator. Several trivial, but ingenious, tips are helpful. Firstly, the optimal length of thread used in laparoscopic suture is between 10 and 15 cm. This length permits the delicate motion for intracorporeal suture by laparoscopic instruments in the limited space. It also helps to keep the appropriate tension during the suturing without loosening of thread or knots that causes the thread to get tangled. Secondly, the type of thread is important. We used 5–0 absorbable multifilament sutures in our model; this suture is characterized by its structure that several suture filaments have been twisted into one thread. This feature would be favorable for the stability of knot during the suture motioning. Additionally, it has the soft and flexible characteristics to better enable knot-making by a novice surgeon.
Regarding the repair method, the stay sutures were on the both edge of CBD anastomosis line at first; then we started to make the repair at the posterior wall. This method could prevent the CBD lumen to become narrow as the anastomosis progressed. The width of CBD lumen was maintained consistently during the anastomosis in our experiments, and operator could feel more comfortable during the operation without the visual interference that helps the suturing more accurately and firmly. After making stay sutures, only 2 additional sutures were needed to make the posterior anastomosis; this procedure resisted the intraluminal pressure and maintained the proper level of flow patency when the bile flow was reopened. The handling of the relatively smaller size of the porcine CBD might pose some technical difficulty. However, we feel that this difficulty in training could act as the advantage rather in the actual clinical situation that could be influenced by a lot of variables. In addition, we inserted the proximal limb of T-tube before placing the distal limb because it is much easier than the insertion of a distal limb before the proximal limb. Authors also suppose that the rubber material is better for the T-tube because it is more flexible than silastic one, which would be helpful to handle or place the tube into CBD.
The surgical outcomes in this article were attained from the limited number of cases. Because one operator could not practice the same experiments repeatedly, we failed to analyze the training effect according to the chronological order. However, even allowing for these limitations, our results about surgical technique seemed to be very favorable and feasible in terms of anastomosis time, the anastomotic security and the completeness of performance especially considering the fact that all operators had no prior experience with this technique. Based on these results, we consider that our training model could act as the effective and reliable practice tool preceded before the implementation of laparoscopic CBD repair with T-tube insertion to the patient in operating room.
In conclusion, the training model for laparoscopic CBD repair using a living porcine model with the application of several tricks as described above could be a feasible training model for a surgeon with no experience in the laparoscopic CBD surgery before. To confirm the training effect of this model in more detail, a further study involving more cases and repeated performance of operation by each trainee should be done.
Figures and Tables
Fig. 2
Intracorporeal view of laparoscopic common bile duct (CBD) transection. (A) CBD was transected by laparoscopic scissors at the middle part about 1 cm in length. (B) The view of transectd CBD which is similar to the common cases of iatrogenic CBD transection.

References
1. Dagash H, Chowdhury M, Pierro A. When can I be proficient in laparoscopic surgery? A systematic review of the evidence. J Pediatr Surg. 2003; 38:720–724.

2. Schoffl H, Hager D, Hinterdorfer C, Dunst KM, Froschauer S, Steiner W, et al. Pulsatile perfused porcine coronary arteries for microvascular training. Ann Plast Surg. 2006; 57:213–216.

3. Pettigrew RA, Burns HJ, Carter DC. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987; 74:791–794.

4. Wu JS, Strasberg SM, Luttmann DR, Meininger TA, Talcott MR, Soper NJ. Laparoscopic hepatic lobectomy in the porcine model. Surg Endosc. 1998; 12:232–235.

5. Hua J, Lin S, Qian D, He Z, Zhang T, Song Z. Primary closure and rate of bile leak following laparoscopic common bile duct exploration via choledochotomy. Dig Surg. 2015; 32:1–8.

6. Cameron BH, O'Regan PJ, Anderson DL. A pig model for advanced laparoscopic biliary procedures. Surg Endosc. 1994; 8:1423–1424.

7. Watson DI, Treacy PJ, Williams JA. Developing a training model for laparoscopic common bile duct surgery. Surg Endosc. 1995; 9:1116–1118.

8. Ferrer J, Scott WE 3rd, Weegman BP, Suszynski TM, Sutherland DE, Hering BJ, et al. Pig pancreas anatomy: implications for pancreas procurement, preservation, and islet isolation. Transplantation. 2008; 86:1503–1510.

9. Strasberg SM, Callery MP, Soper NJ. Laparoscopic surgery of the bile ducts. Gastrointest Endosc Clin N Am. 1996; 6:81–105.

10. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995; 180:101–125.
11. Lee JS, Hong TH. In vivo porcine training model for laparoscopic Roux-en-Y choledochojejunostomy. Ann Surg Treat Res. 2015; 88:306–310.
12. Sanchez A, Otano N, Rodriguez O, Sanchez R, Benitez G, Schweitzer M. Laparoscopic common bile duct exploration four-task training model: construct validity. JSLS. 2012; 16:10–15.

13. Leida Z, Ping B, Shuguang W, Yu H. A randomized comparison of primary closure and T-tube drainage of the common bile duct after laparoscopic choledochotomy. Surg Endosc. 2008; 22:1595–1600.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download