Abstract
Purpose
This study aimed to investigate the incidence and risk factors of early postoperative small bowel obstruction (EPSBO) after laparotomy for trauma patients.
Methods
From 2009 to 2016, consecutive patients who had undergone laparotomy for trauma were retrospectively evaluated. EPSBO was defined as the presence of signs and symptoms of obstruction between postoperative days 7 and 30, or obstruction occurring anytime within 30 days and lasting more 7 days.
Results
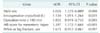
Among 297 patients who met the inclusion criteria, 72 (24.2%) developed EPSBO. The length of hospital stay was significantly longer in patients with EPSBO than in those without EPSBO (median [interquartile range], 34 [21–48] days 24 [14–38] days, P < 0.001). Multivariate logistic analysis identified male sex (adjusted odds ratio [AOR], 3.026; P = 0.008), intraoperative crystalloid (AOR, 1.130; P = 0.031), and Abbreviated Injury Scale (AIS) score for mesenteric injury (AOR, 1.397; P < 0.001) as independent risk factors for EPSBO. The incidence of adhesive small bowel adhesion after 30 days postoperatively did not significantly differ between the 2 groups (with EPSBO, 5.6% without EPSBO, 5.3%; P = 0.571). Most of the patients with EPSBO were recovered by conservative treatment (95.8%).
Conclusion
After laparotomy for trauma patients, the incidence of EPSBO was 24.2% in our study. EPSBO was associated with a longer hospital stay. Male sex, use of intraoperative crystalloid, and AIS score for mesenteric injury were significant independent risk factors for EPSBO. Patients with these risk factors should be followed-up more carefully.
Early postoperative small bowel obstruction (EPSBO) is usually defined as bowel obstruction occurring within 30 days after abdominal surgery [1]. EPSBO affects not only the morbidity but also hospital cost. After abdominal surgery in multiple trauma patients, EPSBO majorly contributes to delaying additional surgery such as orthopedic surgery for accompanying fractures and delaying discharge. Therefore, understanding the clinical features and identifying risk factors of EPSBO are very important for the clinician.
EPSBO should be differentiated from adhesive small bowel obstruction (SBO) which develops at a time remote from the operation because of their different clinicopathological nature [1]. Because the paralytic state lasts 24 hours in the small intestine and 72 hours in the colon after surgery, it is often difficult to differentiate EPSBO from postoperative paralytic ileus [2].
The definition and incidence of EPSBO (0.3%–26.9%) and the characteristics of patients enrolled including nontraumatic patients have varied among several studies [3456789]. Furthermore, the concept of EPSBO, defined as bowel obstruction occurring within 30 days after abdominal surgery, has not been applied in studies of patients undergoing laparotomy after trauma [1011]. For example, Tortella et al. [11] defined early SBO as ileus occurring after a longer period such as 6 months after surgery. The lack of a uniform definition and study design for trauma patients has impaired the understanding of EPSBO in trauma patients. Therefore, the present study aimed to determine the incidence of EPSBO after exploratory laparotomy in trauma patients and to identify the risk factors associated with EPSBO.
After approval from the Institutional Review Board of Chonnam National University Hospital (CNUH-2016-294), consecutive patients who underwent an exploratory laparotomy after a traumatic injury from January 2009 to April 2016 at a tertiary referral trauma center were enrolled in this study. Patients who died within 30 days after surgery, those younger than 15 years, those transferred to another center before oral feeding, and those who underwent laparotomy at another hospital were excluded from the study.
Patients' demographic and clinical data including injury mechanism, age, sex, body mass index (BMI), Glasgow Coma Scale on admission, Injury Severity Score, Abbreviated Injury Scale (AIS) scores for all body regions (head, chest, abdomen, extremity, and pelvis), operative data, and postoperative outcomes were collected and analyzed.
EPSBO was defined as clinical symptoms including nausea, vomiting, and abdominal distension developing within 30 days, and EPSBO was confirmed via imaging studies such as plain abdominal radiography, computed tomography, or contrast study, which can show multiple air fluid levels, distension of the small bowel loops, and the absence of gas in the colon [12]. In order to exclude paralytic ileus (usually 48–72 hours postoperatively), we added the following 2 criteria: (1) the presence of obstructive signs and symptoms at any time within 30 days after the operation and lasting 7 or more days; or (2) the presence of obstructive symptoms of any duration at 7 to 30 days after the operation [3].
The open abdomen was defined as laparotomy abbreviated and fascia left open with temporary abdominal closure (TAC) for damage control surgery [13]. For temporary closure, a sterile 3-L irrigation bag was sutured between the fascial edges. Sodium hyaluronate-carboxymethyl cellulose solution (HA-CMC) (Guardix-sol, 5 g, Hanmi Pharmaceutical, Seoul, Korea) was used as an adhesion-preventing adjuvant (HA-CMC). Before closing the abdominal fascia, HA-CMC solution was sprayed underneath the wound. Superficial or deep surgical site infection (SSI) was defined as skin, subcutaneous tissue, or deep soft tissue infection including one of the following: (1) purulent drainage, (2) diagnosis of superficial or deep SSI by a surgeon, or (3) symptoms of erythema, pain, or edema leading to wound dehiscence [14]. Organ space SSI was defined as purulent drainage or abscess found on direct or radiologic examination [14]. Pancreatic fistula was defined as a drain output of any measurable volume of fluid on or after postoperative day 3 with an amylase content greater than 3 times the serum amylase activity by an international study group (ISGPF) definition [15]. Prolonged weaning was defined as the presence of at least 3 weaning attempts or the need for more than 7 days of weaning after the first spontaneous breathing trial [16]. Complications after surgery were classified according to the recommendations by Dindo et al. [17].
To compare patients with and without EPSBO, univariate analysis was performed. Continuous data are presented as medians with 25th and 75th interquartile range. Categorical data are presented as proportions. Continuous variables were compared using the Mann-Whitney U-test and proportions were compared using chi-square test or Fisher exact test as appropriate.
Logistic regression was used to identify significant risk factors associated with EPSBO. To adjust for confounding factors, variables with a univariate P-value of < 0.10 were included in the multivariate analysis. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
From January 2009 to April 2016, 373 patients underwent exploratory laparotomy after traumatic injury. After excluding patients who died within 30 days (n = 54), patients younger than 15 years (n = 14), patients transferred to another center before oral feeding (n = 7), and patients who underwent laparotomy at another hospital (n = 1), a total of 297 patients were eligible for analysis. The incidence of EPSBO was 24.2% (Table 1). Forty-four patients (14.8%) underwent damage control laparotomy and their fascia was left open with temporary closure for the next definite surgery. The proportion of patients with ileus after 30 days postoperatively (5.4%) was substantially lower than that of patients with EPSBO.
The comparison of the demographic and clinical data of patients with and without EPSBO is shown in Table 2. EPSBO developed more frequently in male patients (88.9% 71.1%, P = 0.002). In patients with EPSBO, the BMI was significantly higher (median [interquartile range], 24.2 [22.0–24.4] kg/m2 22.6 [20.6–24.8] kg/m2, P = 0.004) and the length of hospital stay was significantly longer (34 [21–48] days vs. 24 [14–38] days, P < 0.001) than that in patients without EPSBO. The AIS score for mesenteric injury in patients with EPSBO was significantly higher than that in patients without EPSBO (0 [0–3] 0 [0–0], P < 0.001). Patients with EPSBO tended to have more pelvic fractures or leg fractures than those without EPSBO (37.5% 25.8%, P = 0.055). The overall mortality was 2.0%, and there was no difference in mortality between patients with and without EPSBO (1.4% 2.2%, P = 0.551). There was no difference in the incidence of ileus after 30 days postoperatively between patients with and without EPSBO (5.6% 5.3%, P = 0.571).
Patients' operative data are summarized in Table 3. The use of an HA-CMC was not significantly different between both groups (P = 0.171). There were no between-group differences in gastrointestinal perforation (P = 0.132), intraperitoneal bleeding (P = 0.093), negative laparotomy (P = 0.247), open abdomen (P = 0.099), and previous abdominal surgery (P = 0.309). Mesenteric injury was more frequent in patients with EPSBO (44.4% 17.8%, P < 0.001). The proportion of patients undergoing mesenteric hemostasis was significantly higher among patients with EPSBO (31.9% 16.0%, P = 0.003), while there were no differences in any other operative procedures between the 2 groups. Patients with EPSBO received more intraoperative crystalloid (3,600 [1,900–5,000] mL 2,700 [1,800–4,100] mL, P = 0.033), more intraoperative packed red blood cell (4 [1–8] units 3 [0–6] units, P = 0.024), more fresh frozen plasma (FFP) (2 units [0–4] 0 unit [0–3], P = 0.041), and more peri operative FFP (3 [0–8] units 2 [0–6] units, P = 0.037) than those without EPSBO. The proportion of patients with an operation time ≥180 minutes was significantly higher among patients with EPSBO than among those without EPSBO (26.4% 13.8%, P = 0.013), which might be related to receiving more fluids and blood transfusions.
Patients' postoperative complications are summarized in Table 4. According to the classification by Dindo et al. [17], there were significant differences in postoperative complications bet ween the 2 groups (P < 0.001). The treatment modalities for EPSBO are summarized in Table 5. Most of the patients with EPSBO were successfully recovered by conservative treatment (95.8%), while only 3 patients (4.2%) needed surgical intervention.
Multivariate logistic regression identified three significant risk factors for EPSBO in the present study (Table 6). Male sex (adjusted odds ratio, 3.026; P = 0.008), intraoperative crystalloid (L) (1.130, P = 0.031), and high AIS score for mesenteric injury (1.397, P < 0.001) were independent risk factors for EPSBO.
As far as we know, no study has established a clear definition of EPSBO for only trauma patients undergoing laparotomy. In many cases, the ambiguous symptom of ileus makes its diagnosis difficult. Therefore the incidence of ileus depends on its definition. To clarify the diagnosis, EPSBO was clearly defined according to the definition used in a previous study [3]. In the present study, the incidence of EPSBO was 24.2%, and 1% of the total patient population underwent a surgical intervention to resolve the EPSBO. Most of the patients with EPSBO were recovered by conservative treatment. Patients with EPSBO needed a longer hospital stay. Three parameters (sex, the degree of mesenteric injury, and the use of intraoperative crystalloid) were found to be independent risk factors for EPSBO.
The incidence of EPSBO in the present study (24.2%) is comparable to that reported in previous studies (0.3% to 26.9%); however, it should be noted that the characteristics of the study population, the definition of obstruction, and the duration of follow-up differed between the studies [3456789101118192021]. Clinically, there is no internationally accepted standardized definition for post operative SBO [22]. According to a systematic review and global survey, the time point at which a postoperative ileus becomes prolonged varied (1 day to 7 days) [22]. This means that the concept of physiologic postoperative ileus varies among clinicians. Defining of the time point of postoperative ileus affects its incidence. This also makes the diagnosis of EPSBO difficult.
In addition to adhesion which is a major cause of EPSBO, other possible causes of EPSBO are internal herniation, intussusception, inflammation, and intestinal hematoma [1]. Few studies about postoperative ileus for trauma patients have suggested clinicopathological factors for EPSBO. In a retrospective study of 571 trauma patients by Barmparas et al. [10], the incidence of early in-hospital SBO was 3.9% and only gastrointestinal perforation was found to be an independent risk factor of SBO. The length of hospital stay was longer in the SBO group in this study. The incidence of EPSBO in our study was 24.2%, which is substantially high. Because a clear time point at which a postoperative ileus becomes prolonged was defined in the present study, a larger population may have been diagnosed as having EPSBO. In the present study, a high AIS score for mesenteric injury was a significant risk factor for EPSBO rather than bowel perforation. This result suggested that mesenteric blood flow disturbance which is secondary to mesenteric injury may have an important role in the recovery of bowel motility. Additionally, mesenteric injury is often related to massive bleeding because the mesentery contains affluent blood vessels. This may cause the need of a massive infusion of crystalloid and blood, and may subsequently be related to bowel edema. The length of hospital stay was also longer in patients with EPSBO in the present study. However, we did not find that intraperitoneal contamination caused by bowel perforation had an effect on the presence of EPSBO. Stewart et al. [4] studied 1,072 trauma patients and reported that surgery below the transverse mesocolon caused an increased risk, whereas that in the upper abdomen decreased the risk of EPSBO. In the present study, upper or lower gastrointestinal surgery was not a risk factor. Stewart et al. [4] defined EPSBO as an obstructive symptom after the temporary return of bowel function within 4 weeks of laparotomy and subsequently confirming obstruction at reoperation. By this definition, they reported an incidence of 0.7%. This criterion of diagnosis is more strict than that of our study. This may have contributed to the high incidence of EPSBO in our study. Tortella et al. [11] conducted a prospective study of penetrating abdominal trauma compared with elective surgery and found that small/large bowel penetration or gunshot wounds are at the highest risk and previous abdominal surgery is not a risk for early SBO within 6 months. The proportion of blunt trauma is substantially high in our study because the incidence of blunt trauma in our country is much higher than that of penetrating trauma including gunshot wound. There was no patient with a gunshot wound in our study. Injury type (blunt or penetrating) was not a risk factor in the present study.
In terms of EPSBO in nontrauma patients, Lee et al. [23] found that only history of operation was an independent risk factor of EPSBO including non-trauma colorectal surgery. Additionally, they found that EPSBO was a risk factor for subsequent adhesive SBO in their follow-up study. A study by Kim et al. [24] that included colorectal cancer patient found that male sex, diverting stoma, transfusion, and operation time > 180 minutes were independent risk factors of EPSBO. Long operation time may be associated with the patient's severity, difficulty of the operative technique, receiving more fluids or blood, and more manipulation of the bowel which cause postoperative ileus. In the present study, we found that patient with EPSBO underwent a longer operation (≥180 minutes) and received more blood transfusion (intraoperative and perioperative); however, no statistical significance was found in the multivariate analysis. Additionally, we also found that male sex was an independent risk factor of EPSBO. Few studies reported male sex as an independent risk factor for postoperative ileus after colon surgery [919]. This result may be related to the operative difficulty which was caused by the narrower male pelvis. However, the reason why male trauma patients suffer more from EPSBO is not clear. Vather et al. [9] conducted a prospective study of 327 patients and found that the volume of blood transfusion and crystalloid were risk factors for postoperative ileus. Consistent with this result, we found that the use of intraoperative crystalloid increased the risk of EPSBO. The use of intraoperative crystalloid may cause intestinal edema and decrease bowel motility which contribute to postoperative ileus. More use of intraoperative crystalloid also suggests a more difficult surgery, more severe trauma, and more bleeding which contribute to postoperative ileus.
We assumed that immobilization affects the mesenteric blood flow and recovery of bowel motility after surgery. Therefore, we investigated factors affecting immobilization such as pelvic or leg fracture, and these patients showed a high occurrence of EPSBO. However, we did not find statistical significance in multivariate analysis. Unexpectedly, a previous study revealed that mobilization itself does not shorten the duration of postoperative ileus [25]. To date, there is a lack of evidence that early mobilization enhances the recovery of postoperative ileus.
To date, no study has found increasing EPSBO in patients with an open abdomen. In our study, no association was found between open abdomen and EPSBO. We used a sterile 3-L saline bag for TAC. Recently, several techniques for TAC have been reported including negative pressure wound therapy (NPWT), vacuum pack, Wittmann patch, Bogota bag, and zipper [26]. Although there is increasing evidence of the efficacy of NPWT, a recent systematic review and meta-analysis did not show significantly better outcomes in terms of complications [27].
In the present study, a mixed HA-CMC solution was sprayed underneath the wound to prevent adhesion between the wound and intestine. A significant decrease in the occurrence of postoperative adhesion as shown with the use of this agent [28]. However, the present study did not show any benefit of using HA-CMC solution regarding the incidence of EPSBO. Since HA-CMC was not widely used, adhesion of other parts of the intestine which are not underneath the surgical wound may not have been prevented. In addition to adhesion, other factors such as bowel edema and decreased bowel motility may play a more important role in EPSBO.
This study has several limitations. One important limitation is the retrospective nature of the study. Another limitation is that the selection of the operation method was based on the surgeon's skill level and experience. The small sample size is also an important limitation of this study. This may have contributed more difficulty in detecting real differences in some parameters such as operation time or immobilization. Because of the lack of consensus on the definition of EPSBO, there could be heterogeneity between this study and other studies. Finally, we did not calculate the amount of postoperative opioid use which is also an important risk factor of postoperative ileus. However, the duration of opioid was not a significant risk factor in our study. These concerns may be addressed by a further prospective study or systematic review and meta-analysis.
In conclusion, factors predicting the development of EPSBO were male sex, intraoperative crystalloid fluid, and a high AIS score for mesenteric injury. Patients with these risk factors should be followed-up very carefully. Almost all patients with EPSBO were recovered by conservative treatment. Additionally, the length of hospital stay of patients with EPSBO was longer than that of patients without EPSBO.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI 16 001-1) Chonnam National University Hospital Biomedical Research Institute.
References
3. Pickleman J, Lee RM. The management of patients with suspected early post operative small bowel obstruction. Ann Surg. 1989; 210:216–219.
4. Stewart RM, Page CP, Brender J, Schwesinger W, Eisenhut D. The incidence and risk of early postoperative small bowel obstruction. A cohort study. Am J Surg. 1987; 154:643–647.
5. Quatromoni JC, Rosoff L Sr, Halls JM, Yellin AE. Early postoperative small bowel obstruction. Ann Surg. 1980; 191:72–74.

6. Frykberg ER, Phillips JW. Obstruction of the small bowel in the early postoperative period. South Med J. 1989; 82:169–173.

7. Ellozy SH, Harris MT, Bauer JJ, Gorfine SR, Kreel I. Early postoperative small-bowel obstruction: a prospective evaluation in 242 consecutive abdominal operations. Dis Colon Rectum. 2002; 45:1214–1217.
8. Siporin K, Hiatt JR, Treiman RL. Small bowel obstruction after abdominal aortic surgery. Am Surg. 1993; 59:846–849.
9. Vather R, Josephson R, Jaung R, Robertson J, Bissett I. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery. 2015; 157:764–773.

10. Barmparas G, Branco BC, Schnuriger B, Oliver M, Konstantinidis A, Lustenberger T, et al. In-hospital small bowel obstruction after exploratory laparotomy for trauma. J Trauma. 2011; 71:486–490.

11. Tortella BJ, Lavery RF, Chandrakantan A, Medina D. Incidence and risk factors for early small bowel obstruction after celiotomy for penetrating abdominal trauma. Am Surg. 1995; 61:956–958.
12. Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg. 2013; 8:42.

13. Harvin JA, Wray CJ, Steward J, Lawless RA, McNutt MK, Love JD, et al. Control the damage: morbidity and mortality after emergent trauma laparotomy. Am J Surg. 2016; 212:34–39.

14. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999; 20:250–278.

15. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13.

16. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007; 29:1033–1056.

17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
18. Chapuis PH, Bokey L, Keshava A, Rickard MJ, Stewart P, Young CJ, et al. Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg. 2013; 257:909–915.
19. Millan M, Biondo S, Fraccalvieri D, Frago R, Golda T, Kreisler E. Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg. 2012; 36:179–185.

20. Moghadamyeghaneh Z, Hwang GS, Hanna MH, Phelan M, Carmichael JC, Mills S, et al. Risk factors for prolonged ileus following colon surgery. Surg Endosc. 2016; 30:603–609.

21. Svatek RS, Fisher MB, Williams MB, Matin SF, Kamat AM, Grossman HB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology. 2010; 76:1419–1424.

22. Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013; 17:962–972.

23. Lee SY, Park KJ, Ryoo SB, Oh HK, Choe EK, Heo SC. Early postoperative small bowel obstruction is an independent risk factor for subsequent adhesive small bowel obstruction in patients undergoing open colectomy. World J Surg. 2014; 38:3007–3014.

24. Kim CH, Joo JK, Kim HR, Kim YJ. The incidence and risk of early postoperative small bowel obstruction after laparoscopic resection for colorectal cancer. J Laparoendosc Adv Surg Tech A. 2014; 24:543–549.

25. Waldhausen JH, Schirmer BD. The effect of ambulation on recovery from postoperative ileus. Ann Surg. 1990; 212:671–677.

26. Quyn AJ, Johnston C, Hall D, Chambers A, Arapova N, Ogston S, et al. The open abdomen and temporary abdominal closure systems: historical evolution and systematic review. Colorectal Dis. 2012; 14:e429–e438.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download