Abstract
Purpose
Intra-abdominal adhesions (IAA) are among the most frequently seen pathologies in general surgery practice with an increased morbidity and mortality. In the present study, we investigated the effect of locally applied mesenchymal stem cells (MSCs) on IAA.
Methods
Twenty-four Wistar Albino rats were used in the study. The rats were divided into three groups including: Sham, control, and MSCs group. On day 0, cecum was reached under anesthesia in all groups, except the Sham group. Scraping with a sponge was performed until petechial bleeding occurred. The control group received no treatment. In the stem cell group, MSCs were applied topically immediately after surgery on adhesions. The rats were sacrificed on day 10 and colon tissues and blood samples were collected for macroscopic, histopathological, and biochemical analysis.
Results
In our study, E-selectin, P-selectin, TNF-α and IL-1 levels were statistically significantly lower in the MSC group than the control group, while the sham group has the lowest levels. In both the macroscopic and histopathological analyses (Zühlke's scale), the least amount of adhesion was observed in the Sham group. In addition, although there was less adhesion in the MSC group than the control group, the difference did not reach statistical significance.
Intra-abdominal adhesions (IAAs) are among the more frequently seen pathologies in general surgery practice with an increased morbidity and mortality. While previous operations are mostly held responsible for the adhesions, other factors that promote adhesion formation include inflammation and adhesion-promoting factors found in foreign substances [12].
Fibrous adhesions are, in fact, a response to wound healing in the peritoneal cavity [3]. On the 1st–3rd days of adhesion formation, there are cells coated with fibrin matrix. A majority of the cells are comprised of polymorphonuclear leucocytes. However, they possibly contain peritoneal injury-induced necrotic cells, as well as macrophages, eosinophils, erythrocytes, and tissue debris. On the 4th day, the major cells within the fibrin network are macrophages. From the 5th day, mast cells begin to appear. On the 7th day, the main component of adhesion is collagen and fibroblasts. Small vascular tubes also begin to form. Between the 2nd week and 2nd month, mast cell count increases, whereas almost all of the cells are comprised of macrophage-related collagen fibrils [3].
Simultaneous with formation of the adhesion, a new endothelial layer forms. Various studies concerning new mesothelial cell resources have been conducted [45]. Ellis et al. [6] and Lucas et al. [7] reported that mesothelial regeneration begins with metaplasia of the fibroblasts localized in the loose connective tissue beneath the peritoneum. In electron microscopy examinations, undifferentiated primitive mesenchymal cells were observed in the perivascular connective tissue and these cells were reported to contribute to development of new mesenchymal cells. Then, studies concerning intraperitoneal application of mesenchymal stem cells (MSCs) on IAAs were conducted [8910].
As is well known, stem cells are the cells with self-renewal capacity and the ability to differentiate into more mature cells and different tissue types by asymmetric replication [1112]. These cells can differentiate into the tissue type requiring regeneration once they are concentrated at the damaged tissue area with a cell protective activity in the presence of appropriate inflammatory mediators. Stem cells tissue damage-repairing properties occur by reducing inflammation through their immunomodulatory properties and cytokine release [1314]. Stem cells also play a key role in normal wound healing process. They respond to cytokines released during wound healing and migrate towards the site of inflammation, creating a positive immunity environment [15].
There is limited number of studies on the effectiveness of MSCs on IAA. In the present study, we aimed to investigate the effect of locally applied MSCs on adhesion formation in a rat model.
The study was approved by Kirikkale University, Ethics Committee of Experimental Animals. Twenty-four male Wistar Albino rats were used in the study, with a mean age of 4 months and a mean weight of 240–280 g. All rats were fed with standard feed and water. Feeding was discontinued 12 hours prior to the procedure. The rats were divided into 3 groups including: Sham (n = 8), control (n = 8), and MSCs groups (n = 8).
On day 0, anesthesia consisted of ketamine 90 mg/kg (500 mg/10 mL; Ketalar, Pfizer, Berlin, Germany) and xylazine 10 mg/kg (Rompun, Bayer, Leverkusen, Germany) via intraperitoneal administration. Following anesthesia, abdominal disinfection was performed using povidone iodine, and a midline incision was then performed. The cecum was reached in all groups, except Sham group. It was scraped with a sponge, until petechial bleeding occurred (scraping model) [16]. The control group received no treatment. In the stem cell group, 3 × 106 MSCs were applied topically on adhesions.
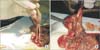
On the 10th day, all rats were euthanized by high dose ketamine. A reverse U shaped incision was made in order to observe entire adhesions and to perform an accurate adhesion grading.
Macroscopic evaluation, histopathological analysis and biochemical analysis were done at day 10.
In this study, MSCs were isolated from subcutaneous flank adipose tissue of rats (Wistar albino, male, 250–300 g). After the xylazine (10 mg/kg; Richter Pharma AG, Wels, Austria) and ketamine (50 mg/kg; Richter Pharma AG) anesthesia of the rats, flank adipose tissue was collected under sterile conditions. The isolation procedure was repeated 3 times with 3 inbreedings of the rats and an average of 0.62 g of adipose tissue was collected per rat (n = 3; n1 = 0.72 g, n2 = 0.57 g, n3=0.57 g). Adipose tissue was transferred into a transport medium DMEM/F12 (Biochrom GmbH, Berlin, Germany) containing 10% (v/v) fetal bovine serum (FBS) (Biochrom GmbH) and 0.4% (v/v) penicillin-streptomycin (Sigma Chemical Co., St. Louis, MO, USA) in a petri dish and then minced into fragments of 4–5 mm in thickness. Tissue fragments were incubated in primary medium (DMEM/F12 containing 20% [v/v] FBS and 0.2% [v/v] penicillin-streptomycin) in a 6-well culture dish under standard culture conditions (in a humidified atmosphere of 95% air and 5% CO2 at 37℃). The culture medium was replaced every day to avoid possible differentiative effects of various cytokines originating from MSCs. Cells were passaged using the standard trypsinization method and, as they were passaged, the cell number was counted using the trypan blue staining method (Sigma Chemical Co.). Trypan blue is a vital staining method used to selectively view blue dead cells. Viable and proliferative cells with intact cell membranes are not colored. In a viable cell, trypan blue is not absorbed. Hence, dead cells are shown with a distinctive blue color under a microscope. The culture medium was removed and the attached cells were harvested using a trypsin/EDTA (0.05%/0.02;w/v) solution (Biochrom GmbH). The cell suspension was then transferred to a centrifuge tube and centrifuged at 800 rpm for 5 minutes to pellet the cells. The supernatant was removed carefully and the cells were incubated in DMEM/F12 containing 10% (v/v) FBS and 0.2% (v/v) penicillin-streptomycin. The MSCs were passaged 4 times and then cryopreserved for future applications. For cryopreservation, cells were detached from the surface of the culture dish by using a trypsin/EDTA solution. The cell suspension was immediately transferred into a freezing medium containing 50% (v/v) FBS, 40% DMEM/F12, and 10% (v/v) DMSO (Sigma Chemical Co.) in a cryogenic tube. Cryovials were stored at −80℃ for 48 hours and then the frozen vials were transferred to a liquid nitrogen container at −196℃ for long-term storage. For standardization of cell concentrations for future applications, all cells harvested from the three 6-well culture dishes were collected in 1 cryogenic tube. Cell morphology and growth were examined under an inverted microscope (IX70, Olympus, Tokyo, Japan).
MSCs were characterized using immunofluorescence staining of CD13 and CD29 molecules (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) during their second passage. For immunostaining, cells grown in culture dishes were washed in PBS (Biochrom GmbH) and fixed for 5 minutes in methanol at −10℃. After fixation, the methanol was removed and desiccated. For blocking, the cells were incubated for 20 minutes with blocking serum (normal goat serum). After blocking, the blocking serum was removed, and the cells were washed 3 times in PBS and then incubated for 1 hour with primary antibody for CD13 and CD29 molecules. After washing for 5 minutes in PBS, the cells were incubated for 45 minutes with a secondary antibody (donkey antigoat Ig-TR) and washed 3 times in PBS. After washing, the cells were mounted with a mounting medium and visualized under fluorescence microscope (BH2-RFL-T3 model fluorescence attachment, Olympus). All procedures were done at room temperature. The flow cytometry analysis was done against CD29, CD90, CD54, MHC Class I, CD45, CD106, and MHC Class II for characterization of MSCs. Flow cytometry analysis was performed [17].
Blood samples were collected and centrifuged at 3,000×g for 10 minutes to obtain the serum. All samples were stored at −80℃ until analysis. In the analysis of these samples, E-selectin, P-selectin, TNF-α, and IL-1β levels were measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Multiskan FC, 2011-06, Thermo Fisher Scientific, Waltham, MA, USA).
The total protein amount of the samples was determined using the Bradford assay kit (Thermo scientific Pierce BCA) and was examined at 595-nm wavelength with a plate reader (Multiskan FC, 2011-06).
ELISA Kit: The samples were thawed and ELISA kits (Eastbiopharm Co. Ltd., Hangzhou, China) were used for the quantitative measurements of malondialdehite. The samples and standards were placed to microplate wells, which are precoated with an anti-human monoclonal antibody prior to incubation and, then, biotin was added to each well combined with Streptavidin-HRP to form the immune complex. All were incubated and washed to remove the uncombined enzymes. Chromogen solution A and B were added to each well, and the color of the liquid changed to blue. By adding the stop solution, an acid effect, the color finally became yellow. Optical density was read on a standard automated plate reader (Multiskan FC, 2011-06) at 450 nm.
Statistical analysis was performed using the SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed in mean ± standard error. Both macroscopic and microscopic adhesion classification grades were presented in numbers (i.e., grade 0 = 0, grade 1 = 1, grade 2 = 2, grade 3 = 3, and grade 4 = 4). Due to the limited number of rats in each group, nonparametric methods were used for statistical analysis. For intergroup analysis, the Kruskal-Wallis variance analysis was used to analyze significant differences among the groups. A P-value of less than 0.05 was considered statistically significant.
The results of the biochemical, microscopic, and macroscopic analysis of IAAs are presented in Table 3.
In our study, E-selectin levels showed significantly lower levels in MSC group than control group. Compared to MSC group, Sham group had statistically significantly lower E-selectin levels. In addition, P-selectin levels were significantly lower in MSC group than control group, while these levels in Sham group were significantly lower than MSC and control groups. TNF-α and IL-1 levels were statistically significantly lower in Sham group than MSC and control groups, while both levels were statistically significantly lower in MSC group than the control group.
In the macroscopic evaluation, there was statistically significant difference between Sham and control groups. In addition, although there was less adhesion in MSC group than the control group, the difference did not reach statistical significance.
In the histopathological analysis (Zühlke's scale), the least amount of adhesion was observed in Sham group, indicating a statistically significant difference among Sham, MSC, and control groups. In addition, although there was less adhesion in MSC group than the control group, the difference did not reach statistical significance.
Numerous studies have been performed to decrease IAAs [202122]. The attempted approaches to decrease adhesion include decreasing the inflammatory response, increasing fibrin lysis, decreasing fibrin formation, or prevention of the injured serosa.
In a study by Lucas et al. [8] on how stem cell use decreases the rate of IAA formation, the use of stem cells decreased adhesion in groups formed by injection of 1.4×106 MSCs and 5×106 MSCs given immediately after surgery, with a high efficacy in the latter group. Yet, MSCs injected 4–6 hours after surgery increased the adhesion scores. However, a more recent study has suggested that intraperitoneal MSC use 24 hours after injury does not have a reducing effect on the IAA formation; but if it is injected via tail vein MSCs may attenuate peritoneal injury [9]. These aforementioned studies used MSCs with the tail vein; however, they showed that MSCs accumulated in the lung, spleen, and liver, but not in the injured peritoneum, even if they were used intraperitoneally. The reason behind the absence of MSCs in the injured peritoneum could be the phagocytic response of the monocyte-macrophage system.
In another study conducted by Wang et al. [10], the effectiveness of MSCs intraperitoneally applied at 0, 4th, 12th, 24th, and 48th hours was compared and not found to be effective on IAA.
Differing from the aforementioned studies, we applied MSCs topically immediately after the formation of adhesion. We are of the thought that local and early stem cell application is a more effective method in reducing IAAs. We are also of the thought that the reason for this is that the increase in phagocytic cells responsible for development of adhesions does not occur yet owing to direct administration on the lesion and is a direct interaction of MSC with the damaged area.
In the histopathological and macroscopic examinations, adhesion formation in MSC group was less than the control group; however, it did not reach statistical significance. This can be explained by the small sample size in each group.
To assess the response to adhesion formation various serum markers (selectins, TNF-α, and IL-1 levels) were evaluated. Selectins, which are cell-surface glycoproteins, are the members of the family of adhesion molecules, playing a role in capture, rolling, and slow-rolling stages which occur during the accumulation of polymorphonuclear leukocytes at the site of injury [2324]. There are also evidences from animal studies showing that P-selectin plays a central role in the binding of neutrophils to the endothelium activated at the early stage and spontaneous rolling of neutrophils within the postcapillary venules [24]. E-selectin is found in endothelial cells and rapidly released from these cells in response to inflammatory cytokines [2324]. The level of selectins is associated with the progression of the inflammatory process [23]. TNF-α and IL-1 are also one of the main causes of IAAs.
While the adhesion-reducing effect of MSCs was not able to be clearly shown in the present study, it could be due to the anti-inflammatory effect of MSCs which was revealed by the decline in TNF-α and IL-1 levels. The decline in P-selectin and E-selectin levels suggest that MSCs induce the migration of leukocytes, an important step in adhesion formation, to the injured area and decrease adhesion to this area thereby reducing the inflammatory process and IAAs, eventually.
In contrast to our study, inflammatory markers have not been evaluated in previous studies investigating the effectiveness of MSC on IAAs. In this study, we managed to reveal the anti-inflammatory effectiveness of the topical application of MSC on an IAA.
In conclusion, topical MSC application immediately after surgery suppresses the inflammatory process. However, it cannot be found to be effective in histopathological and macroscopic examinations performed on the 10th day. There is a need for controls performed at later periods and studies conducted in larger groups on this issue.
Figures and Tables
References
1. Drollette CM, Badawy SZ. Pathophysiology of pelvic adhesions. Modern trends in preventing infertility. J Reprod Med. 1992; 37:107–121.
3. diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001; 7:547–555.

4. Brunschwig A, Robbins GF. Regeneration of peritoneum: experimental observations and clinical experience in radical resections of intra-abdominal cancer. In : XVth Congress of the Society of International Chirurgie; Lisbonne. Bruxelles: Henri de Smedt. 1953. p. 756–765.
6. Ellıs H, Harrıson W, Hugh TB. The healıng of perıtneum under normal and pathologıcal condıtıons. Br J Surg. 1965; 52:471–476.
7. Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res. 1996; 65:135–138.
8. Lucas PA, Warejcka DJ, Zhang LM, Newman WH, Young HE. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 1996; 62:229–232.

9. Wang N, Li Q, Zhang L, Lin H, Hu J, Li D, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012; 7:e43768.

10. Wang N, Shao Y, Mei Y, Zhang L, Li Q, Li D, et al. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res Ther. 2012; 3:51.

11. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000; 100:157–168.
12. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005; 2:8.
13. da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008; 26:2287–2299.

14. Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesen chymal stem cells derived from adult human tissues. Cell Immunol. 2009; 259:150–156.
15. Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for atte nua tion of scar formation during wound healing. Stem Cell Res Ther. 2012; 3:20.
16. Elkins TE, Bury RJ, Ritter JL, Ling FW, Ahokas RA, Homsey CA, et al. Adhesion pre ven tion by solutions of sodium carboxy methylcellulose in the rat. I. Fertil Steril. 1984; 41:926–928.
17. Niyaz M, Gurpınar OA, Gunaydın S, Onur MA. Isolation, culturing and characterization of rat adipose tissue derived mesenchymal stem cells: a simple technique. Turk J Biol. 2012; 36:658–664.
18. Nair SK, Bhat IK, Aurora AL. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch Surg. 1974; 108:849–853.

19. Zuhlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990; 1009–1016.
20. Müller SA, Treutner KH, Haase G, Kinzel S, Tietze L, Schumpelick V. Effect of intraperitoneal antiadhesive fluids in a rat peri tonitis model. Arch Surg. 2003; 138:286–290.
21. Tarhan OR, Barut I, Sutcu R, Akdeniz Y, Akturk O. Pentoxifylline, a methyl xanthine derivative, reduces peritoneal ad hesions and increases peritoneal fibrinolysis in rats. Tohoku J Exp Med. 2006; 209:249–255.
22. Aysan E, Basak F, Kinaci E, Yanar H, Coskun H. Experimental adhesion model: effect of viscosities of fluids put in the peri toneal cavity on preventing peritoneal adhesions. Exp Anim. 2007; 56:349–354.
24. Gao Y, Li N, Fei R, Chen Z, Zheng S, Zeng X. P-Selectin-mediated acute inflammation can be blocked by chemically modified heparin, RO-heparin. Mol Cells. 2005; 19:350–355.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download