Abstract
Gastrointestinal (GI) tract metastasis of primary breast cancer is very rare. We present a patient with small bowel obstruction from distant metastasis of primary breast cancer. Each characteristic features of concern of GI tract distant metastasis from many pervious studies has been reported differently. We should remember that GI tract metastasis may coexist when patients with breast cancer have intermittent or recurrent abdominal pain with or without obstructive symptoms.
A major cause of death in breast cancer patients is distant meta stasis. The most common sites of distant metastasis in breast cancer patients are lungs, liver, bones, soft tissue, and adrenal glands [1]. However, involvement of the gastrointestinal (GI) tract is very rare. After analyzing more than 2,500 cases of breast cancer with metastatic disease over an 18-year period, Borst and Ingold [2] reported that only 17 patients (0.68%) were found to have metastases to the GI tract. McLemore et al. [3] reported that only 23 cases (0.19%) had GI metastasis alone out of the 12,001 cases of distant breast metastasis over 15 years.
In Korea, Son et al. [4] reported that during the median follow-up period of 45 months, 375 patients out of the 3,700 patients (10.1%) developed distant recurrence. The percentages of additionally reported common sites of distant metastasis were in the following sequential order: multiple sites, 29%; bone, 26.4%; lungs, 23.8%; liver, 8%; and brain, 4%. Only a few cases of GI tract metastasis from breast cancer patients have been reported in Korea to date [56].
We report a rare experience of solitary small bowel metastasis from primary breast cancer in Korea.
A 56-year-old woman visited the Emergency Department of Ajou University Hospital due to cramp-like intermittent abdominal pain with nausea and vomiting symptoms over the last 2 months. She had a past history of infiltrating lobular carcinoma in her right breast. She was included in a clinical study, when she was diagnosed with breast cancer, and had previously received neoadjuvant endocrine therapy with letrozole (letrozole 2.5 mg once a day) and metformin (500 mg) for 6 months. She was part of a control group, which we know through her prescribed medicines. The clinical stage before neoadjuvant chemotherapy was cT4N0M0-stage IIIB. The initial tumor was 5.4 cm in size with direct skin invasion without lymph node or distant metastasis. estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) status was positive, positive, negative, respectively, in core biopsy. After neoadjuvant therapy, she underwent right total mastectomy with sentinel lymph node biopsy one year prior to the Emergency Department visit. At that time, the pathologic TNM stage was ypT3N0(i) M0-stage IIB and hormone receptor status was ER positive, PR positive, and HER2 negative. After she received chemotherapy and radiation therapy, she took letrozole 2.5 mg once a day (daily) for 4 months before her visit to the Emergency Department of Ajou University Hospital. She underwent abdominal CT and the result showed persistent short segmental bowel wall thickening in the distal jejunum (Fig. 1). However, she complained of passage of intestinal gas and had loose stools without melena. She was suspected to have partial small bowel obstruction. She was admitted and enteroscopy and capsule endoscopy were performed to rule out a mass or inflammatory change in the obstructive lesion. Enteroscopy results were clear and no definite mucosal lesion could be found from the esophagus to the proximal ileum although the enteroscope passed through the suspected jejunal lesion. However, the capsule endoscope could not pass through the lesion and diffuse thickening and an erythematous mucosal change were noted. Finally, surgical assessment was performed. She underwent elective laparotomy and obstructive jejunum segmental resection and jejunojejunostomy were performed. No mesenteric lymph node metastasis was found. The specimen was 4.4 cm in length and the mucosa of the obstructive lesion showed an ill-defined, slightly elevated lesion measuring 1.7 cm with focally thickened wall measuring 1 cm in thickness (Fig. 2). Microscopic examination showed that the mucosal layer was intact and infiltrating lobular carcinoma spread from the serosal layer to the submucosal layer on Hematoxylin and Eosin (H&E) staining (Figs. 3, 4, 5). Additionally, multiple tumor emboli were observed in the lymphovascular spaces. On immunohistochemistry, the tumor cells were positive for ER and negative for PR, and HER2 staining was negative.
The patient was discharged 6 days after surgery without complications. She revisited the outpatient clinic in good condition and was examined by PET-CT for detection of additional distant metastasis. There were no other distant metastatic lesions. Finally, she was recommended continual endocrine therapy.
Distant metastasis of breast cancer is commonly found in bones, lungs, and liver, but not in the GI tract [1234].
The incidence of GI tract metastasis of breast cancer in various reports was not more than 1 percent [23]. However, in an autopsy study of breast cancer death, a rate of 16% for GI tract metastasis was detected; this rate was quite different from that in a retrospective study [1]. There were also in differences of developmental lesion in the GI tract distant metastases. In an analysis of 12,000 breast cancer patients with distant metastasis over 15 years, 53 patients had only GI tract metastasis; the number of cases with esophageal metastasis was 4 (8%), the number of cases with small intestinal metastasis was 10 (19%), the number of cases with gastric metastasis was 15 (28%), and the number of cases with colon and rectal metastasis was 24 (45%). As per these results, small bowel metastasis is one of the more rare presentations of GI tract metastases [3]. However, in an autopsy study of breast cancer death, the rate of gastric metastasis was 10%, the rate of small bowel metastasis was 9%, and the rate of colon metastasis was 8% [1]. There were differences between the retrospective study and the autopsy study, and the autopsy study results showed a higher value of statistics in small bowel distant metastasis than in colonic metastasis.
The interval between the diagnosis of primary breast cancer and distant GI metastasis was very different in each study. In an analysis of 12,000 breast cancer patients with distant metastasis over 15 years, the mean interval was 7 years [3]. Another study showed that the mean interval was 4 years with the longest interval at 28 years [57]. Generally, many reports showed a mean interval of at least more than 2 years, but in our case, the interval was 18 months, shorter than that in the other studies.
The clinical symptoms of GI tract metastasis vary on a case-by-case basis from no signs to severe obstructive features. McLemore et al. [3] reported that the most common signs and symptoms were abdominal pain with dyspepsia, followed by bloating, melena, GI hemorrhage, bowel obstruction, nausea and vomiting, early satiety, dysphagia, weight loss, anemia or fatigue, and a palpable mass. Our patient presented with partial small bowel obstruction with intermittent abdominal pain. They also reported that the diagnostic difficulty in patients with GI tract metastasis is demonstrated by the fact that 21% of patients were thought to have a different disease and 11% of patients did not receive a diagnosis until they underwent an exploratory laparotomy [3]. In our case, in spite of the endoscopic approach, we failed to detect metastasis because the obstructive mucosal lesion was intact which we confirmed based on the final pathologic results. In this regard, a surgical approach was appropriate for diagnosis and treatment.
It is well known that invasive lobular carcinoma has a higher prevalence of visceral metastasis or GI tract metastasis than invasive ductal carcinoma [8]. McLemore et al. [3] reported that GI metastases in patients with invasive lobular carcinoma outnumbered 2 fold those found in patients with invasive ductal carcinoma. On comparison of the clinical metastatic patterns of invasive lobular and ductal carcinoma of the breast, Dixon et al. [9] reported that peritoneal metastases were detected significantly more often in invasive lobular carcinoma (P = 0.0003). Our patient also had invasive lobular carcinoma on histopathology. On pathological diagnosis, it is difficult to identify GI tract metastasis or primary carcinoma of GI mucosal origin. Immunohistochemical (IHC) assay such as ER and PR staining can be helpful in the differential diagnosis of GI metastasis of breast cancer origin. If there is suspected GI metastasis from breast cancer but tumor cells do not stain positive for ER and PR, gross cystic disease fluid protein-15 (GCDFP-15) staining also can be helpful [10]. In our patient, tumor cells were positive for ER and negative for PR and HER2 on IHC staining, and this result was similar to the primary breast cancer hormone receptor status which revealed small bowel distant metastasis of primary breast cancer origin.
There are various therapeutic methods of treating GI tract metastasis [3]. Systemic chemotherapy, endocrine therapy, or surgical intervention can be adopted, but there is a lack of adequate long-term follow-up data of survival and recurrence. McLemore et al. [3] reported that patients with GI metastasis only who underwent palliative surgical intervention tended to have a more prolonged median survival (44 months vs. 9 months). But this difference was not statistically significant.
In spite of statistical failure, this result showed that surgical intervention could be attempted for better survival benefits after eliminating carcinoma when there is single or curable GI tract metastasis. We need more evidence for the surgical benefits of single GI tract metastasis from long-term follow-up data. Palliative surgery could also be attempted for relief of symptoms in incurable cases or surgical abdomen. Our case showed single small bowel metastasis for which we decided to perform observation with endocrine therapy after surgery.
GI tract distant metastasis is not a frequently occurring condition, and it is difficult to differentiate this entity from other diseases. We must remember that GI tract metastasis may be likely when patients with breast cancer, especially invasive lobular carcinoma, have intermittent or recurrent abdominal pain with or without obstructive symptoms.
Figures and Tables
Fig. 1
Abdominal CT shown persistent short segmental bowel wall thickening in the distal jejunum (left upper quadrant of the abdomen).
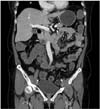
Fig. 2
An obstructive jejunal lesion measuring 4.4 cm in length. The mucosa showed an ill-defined, slightly elevated lesion measuring 1.7 cm and the wall was focally thickened measuring 1 cm in thickness.

Fig. 3
Microscopic findings of carcinoma cells that infiltrated from the serosal layer to the submucosal layer. Mucosal layer was intact (H&E, ×40).

References
1. Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979; 11:193–205.

2. Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993; 114:637–641.
3. McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005; 12:886–894.

4. Son BH, Ahn SH, Kwak BS, Kim JK, Kim HJ, Hong SJ, et al. The recurrence rate, risk factors and recurrence patterns after surgery in 3700 patients with operable breast cancer. J Breast Cancer. 2006; 9:134–144.

5. Park KH, Bae JS, Chae BD, Hong KM, Lee CW, Lee SI. Solitary duodenal metastasis from breast cancer. J Korean Surg Soc. 2008; 74:76–78.
6. Choi JE, Park SY, Jeon MH, Kang SH, Lee SJ, Bae YK, et al. Solitary small bowel metastasis from breast cancer. J Breast Cancer. 2011; 14:69–71.

7. Theraux J, Bretagnol F, Guedj N, Cazals-Hatem D, Panis Y. Colorectal breast carcinoma metastasis diagnosed as an obstructive colonic primary tumor. A case report and review of the literature. Gastroenterol Clin Biol. 2009; 33:1114–1114.
8. Bamias A, Baltayiannis G, Kamina S, Fatouros M, Lymperopoulos E, Agnanti N, et al. Rectal metastases from lobular carcinoma of the breast: report of a case and literature review. Ann Oncol. 2001; 12:715–718.

9. Dixon AR, Ellis IO, Elston CW, Blamey RW. A comparison of the clinical metastatic patterns of invasive lobular and ductal carcinomas of the breast. Br J Cancer. 1991; 63:634–635.

10. Mazoujian G, Pinkus GS, Davis S, Haagensen DE Jr. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast car ci no mas with apocrine features. Am J Pathol. 1983; 110:105–112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download