Abstract
Purpose
Recent studies investigating new strategies to modulate the immune system have utilized animal models of liver transplantation (LT). However, the anhepatic phase (AHP) remains a crucial problem in LT. The aim of the present study is to introduce a technique for successful orthotopic LT in cynomolgus monkeys using an early-reperfusion strategy.
Results
In 2 recipients, liver allografts were perfused after suprahepatic inferior vena cava (SHIVC), portal vein (PV), and infrahepatic inferior vena cava (IHIVC) anastomosis. To reduce the time of AHP in five recipients, liver allografts ware perfused after SHIVC and PV anastomosis while the IHIVC was not anastomosed. In the latter strategy, the AHP was reduced from 46 minutes to 31 minutes and a 24-hour survival rate of 80% was achieved.
Acquiring immunological tolerance has been a major issue in the transplantation field for decades. Experiments in rodents and nonhuman primates (NHP) have improved our understanding of this phenomenon [1234]. However, further studies are necessary to achieve immunological tolerance in a clinical setting. Due to the presence of resident regulatory cells and cytokines, the liver is the most tolerogenic solid organ with comparably lower rejection rates and immunosuppressant requirements [5]. Based on this phenomenon, recent studies have utilized animal models of liver transplantation (LT) to investigate new strategies to modulate the immune system [236]. However, intestinal congestion during the anhepatic phase of LT remains a crucial problem for achieving reliable survival of animal models. Previously, superior mesenteric artery (SMA) clamping [7] and an active porto-caval shunt [8] have been introduced as solutions. However, porto-caval shunts are difficult to use in cynomolgus monkeys because of animal size and lack of proper instrumentation, while SMA clamping can induce ischemic damage to additional organs including the pancreas, duodenum, and jejunum. Therefore, in the present study, we aimed to introduce a method for LT in NHPs using an early-reperfusion strategy.
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2011) and the Animal Welfare Act (2008) in the animal facility of the Nonhuman Organ Transplantation Research Center at Genia Inc. (Seongnam, Korea). The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Genia Inc. (ORIENT-IACUC-16052).
Acynomolgus monkey between 3.6 and 5 kg was sedated with an intramuscular (IM) injection of ketamine (7 mg/kg) and transferred from its cage to the operating table. Vital signs including blood pressure, pulse rate, respiration rate, body temperature, and oxygen saturation were monitored. The donor monkey was anesthetized with isoflurane using a facial mask (3% for induction, 1% for maintenance during the operation) and its muscles were relaxed with an intravenous (IV) injection of rocuronium bromide (0.3 mg/kg). Endotracheal intubation was performed with a 3.5F. endotracheal tube. An eye ointment was used to prevent dryness while under anesthesia. The abdominal wall was sterilized and covered with sterile drapes. A long midline laparotomy was performed to expose the liver and abdominal aorta at the iliac bifurcation. The falciform ligament and triangular ligament was divided using electrocautery. The liver was released from the diaphragm and the infrahepatic inferior vena cava (IHIVC) was dissected down to the level of the renal vein. The portal vein (PV), common bile duct (CBD), and hepatic artery (HA) were separated from the hepatoduodenal ligament. The HA was dissected down to the celiac truck. The gastroduodenal artery, right gastric arteries, left gastric artery, and splenic artery were divided between ties. The aorta was dissected down to the iliac bifurcation. Renal arteries and spinal arteries were divided between ties. Ties were set around the superior and inferior mesenteric arteries, and left for in situ liver perfusion through the intestinal vasculature. The gallbladder was removed and any bleeding from the gallbladder bed was cauterized. In situ liver perfusion was performed with 500 mL of cold histidine-tryptophan-ketoglutarate (HTK) solution through the aorta just after clamping both iliac arteries and the supraceliac aorta, and cutting the suprahepatic inferior vena cava (SHIVC) and IHIVC. The superior and inferior mesenteric artery was ligated with preset ties and divided. The supraceliac aorta was ligated with a tie and divided just above the ligation point. The abdominal aorta at the insertion site for the perfusion catheter was divided. The CBD, PV, SHIVC, and IHIVC, were divided leaving all remaining vessels long. The liver was extracted and placed into a sterile organ bag on ice. The diaphragmatic patch surrounding the SHIVC was trimmed and all phrenic veins were closed.
Anesthesia was induced in the same manner as in the donor monkey. The animal was placed in the left lateral decubitus position and the neck was sterilized for infusion port insertion. A 2-cm-sized neck incision was made at the site where the carotid arterial pulse was detected. The internal jugular vein was exposed and the catheter of the infusion port was inserted in. The port was placed on the back of the monkey to prevent possible damage by the monkey's hand. The animal was placed in the supine position. The abdominal wall was sterilized and covered with sterile drapes. A long midline laparotomy was performed to expose the liver and abdominal aorta at the iliac bifurcation. The falciform ligament and triangular ligament were divided using electro-cautery. The liver was released from the diaphragm. The SHIVC was dissected from the diaphragm and the IHIVC was dissected down to the level of the renal vein. The IHIVC and abdominal aorta were exposed by Kocherization to facilitate simple IHIVC anastomosis and graft aorta conduit anastomosis with the recipient abdominal aorta. The PV, CBD, and HA were separated from the hepatoduodenal ligament. Heparin (50 IU/kg) was given through the IV line 3 minutes before clamping the vessels. The CBD was clamped with a bulldog clamp and divided at the liver hilum. The HA was ligated at the hilum of the liver. The PV was clamped with a bulldog clamp. The IHIVC was clamped with a vascular clamp and firm pressure was applied onto the liver to remove a portion of the remaining blood. The SHIVC was clamped with a vascular clamp. The HA, PV, SHIVC, and IHIVC were divided as close to the liver as possible. The recipient liver was removed from the peritoneal cavity and isoflurane concentration was adjusted according to the arterial pressure and monkey's reactivity. Methylprednisolone (20 mg/kg) was given to initialize immunosuppression. The donor liver was placed into the peritoneal cavity. The donor SHIVC was trimmed to fit the recipient side. Both ostia of the recipient and donor SHIVC were approximated. Right and left side corner sutures were made 180° apart using 6-0 monofilament polypropylene sutures. Ties on both sides with corner sutures were placed and continuous sutures were made towards each other. A PV anastomosis was created using 6-0 monofilament polypropylene sutures in the same manner, except leaving approximately 0.5 cm of growth factor. The PV was unclamped to wash out the HTK solution in the graft and congested blood in mesenteric veins. Next, the IHIVC was clamped and the SHIVC was unclamped to perfuse the graft liver. IL-2 receptor antagonist (simulect, 10 mg), protease inhibitor (gabexate, 20 µg/kg), prostaglandin E2 (2.95 µg/kg), and low molecular weight heparin (dalteparin, 50 IU/kg) were given. An IHIVC anastomosis was made in the same manner as for the SHIVC anastomosis. Before finishing the anastomosis, the recipient side IHIVC was unclamped to wash out systemic acid metabolites coming from the lower extremities. The graft aorta conduit was placed on the retroperitoneal space along the IHIVC. Back bleeding from the donor aorta was assessed. The donor aorta was flushed with 10 mL of heparinized saline and a bulldog clamp was placed distally. The abdominal aorta below the inferior mesenteric artery was clamped to avoid additional intestinal ischemia. A 10-mm incision was made on the anterior wall of the clamped abdominal aorta. An end-to-side anastomosis with interrupt sutures was made between the donor and recipient aorta using 8-0 monofilament nylon sutures. The bile duct was anastomosed in an end-to-end manner with interrupt sutures using 7-0 monofilament polypropylene sutures. After checking for hemostasis, the abdominal wall was closed with a layer-by-layer technique using 4-0 braided polyglycolic-acid sutures for the fascia layer and 4-0 monofilament nylon sutures for the skin layer.
Anesthesia was weaned and mechanical ventilation was stopped when the monkey was able to breathe independently. The endotracheal tube was removed when the monkey's breathing stabilized. The monkey was moved back to its cage when it was able to move its arms and legs. Normal saline 10 mL/hr was given through the infusion port with 20 µg/kg of protease inhibitor (gabexate), 2.95 µg/kg of prostaglandin E1, and 50 IU/kg of low molecular weight heparin (dalteparin) for 2 weeks. IL-2 receptor antagonist (simulect, IV, 10 mg) was given at postoperative day 4. Tacrolimus was given once a day (IM, 0.1 mg/kg, target trough level 8–10 ng/mL) and mycophenolic acid (250 mg) was given orally twice a day. Methylprednisolone was tapered to a dose of 1 mg/kg over seven days, and was changed to oral prednisone thereafter (0.3 mg/kg). Vascular structures were assessed with Doppler ultrasonography at postoperative days 1, 3, 5, and 7, and weekly thereafter.
The LT technique described above was successfully performed using cynomolgus monkeys. The results of this study are summarized in Table 1. In 2 cases, reperfusion was performed after IHIVC anastomosis, consistent with human LT. However, none of these monkeys were able to wean from anesthesia. Conversely, in 5 cases in which reperfusion was performed after SHIVC and PV anastomosis while the IHIVC was not anastomosed (Figs. 1, 2), 4 of the monkeys (4 of 5, 80%) were able to wean from anesthesia while 1 monkey died on the operating table as a result of pneumothorax from a traumatic diaphragm rupture. Two of the animals survived for more than 1 week under cover of maintenance immunosuppression.
Lastly, using the early-reperfusion strategy, the median anhepatic phase time (AHPT) was reduced from 46–31 minutes (P = 0.04).
The development of immunosuppressive drugs has opened the door to organ transplantation, and many investigators are working on developing new immunosuppressive drug protocols capable of providing further improvement to the outcomes of transplant patients and reducing tissue toxicity. However, despite these efforts, all of the currently available immunosuppressive drugs have serious side effects including nephrotoxicity, development of malignancies, and increasing susceptibility to infection by opportunistic pathogens [910]. Thus, many investigators are now focusing on identifying methods to decrease or eliminate the need for immunosuppressive drugs. Owing to their similar immunologic characteristics with humans, many of these experimental studies are performed using nonhuman primates (NHPs) [411], mainly in the setting of kidney transplantation. However, the liver is the most immune-regulatory solid organ that is transplanted, and thus tolerance in LT can be achieved more easily [5]. Therefore, in the present study, we tried to establish a LT model using NHPs.
During AHPT in LT using NHPs, it is necessary to block blood flow in 2 ways, namely, PV flow from the mesenteric veins and vena cava flow from the lower extremities. Since the inferior vena cava (IVC) is embedded in the liver parenchyma in NHPs, an IVC replacement technique is inevitable. However, during IVC reconstruction, blood flow from the lower extremities is blocked, and since collateral circulation of the PV is not developed in healthy NHPs, blood flow from mesenteric veins is also blocked. To solve this problem, veno-veno bypass has been successfully used in previous studies [812]. However, veno-veno bypass is difficult to perform in NHPs because of the small size of vessels. Oura et al. [7] performed NHP LT using an SMA clamping technique, and reported that early-reperfusion technique without SMA clamping yields a 24-hour survival rate of 33.3% (3 of 9). In their study, the AHPT was longer than 40 minutes in both groups. However, in the present study, the median AHPT was 31 minutes and the 24-hour survival rate was 80% (4 of 5) in the early-reperfusion group. Therefore, our results suggest that an AHPT of less than 35 minutes can be tolerated in NHPs.
To reduce the complexity of performing anastomoses and reduce operating time, it is important to clearly expose the IVC on the recipient side. Obtaining this exposure and opening the space where the graft aorta conduit is placed can be achieved using Kocherization. This technique also renders the surgical field shallower, enabling surgeons to move their hands more easily.
There are several limitations of this study. We could not avoid the bias from the proficiency. However, the operator had already had sufficient experience of kidney transplantation in cynomolgus monkeys and LT in human. Three cases including all cases of non-early reperfusion did not have survived for 24 hours after LT. Therefore, comparing immediate postoperative liver function between 2 groups was impossible. One recipient showed normal liver enzyme level (normal range: AST, 20–57 mg/dL; ALT, 23–61 mg/dL) 14 days after LT. We are planning to follow up the liver vascular structure with Doppler ultrasonography based on the previously reported normal findings [13].
In conclusion, the LT model introduced in this study with cynomolgus monkeys represents a promising tool for transplantation research, and can be applied using the technique described.
Figures and Tables
Fig. 1
Scheme of the early-reperfusion strategy. (A) PVs, SHIVCs, and IHIVCs were prepared for anastomosis. (B) Reperfusion was performed after SHIVC and PV anastomosis while the IHIVC was not anastomosed. (C) IHIVC anastomosis was performed after reperfusion. SHIVC, suprahepatic inferior vena cava; IHIVC, infrahepatic inferior vena cava; PV, portal vein.

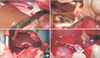
Fig. 2
Actual photos of liver transplantation using early-reperfusion strategy. (A) SHIVC anastomosis was performed first. (B) PV anastomosis was performed in succession. (C) Reperfusion was performed after SHIVC and PV anastomosis while the IHIVC was not anastomosed. Note the difference of liver surface color before and after reperfusion. (D) IHIVC anastomosis was performed after reperfusion. SHIVC, suprahepatic inferior vena cava; IHIVC, infrahepatic inferior vena cava; PV, portal vein.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C2859).
References
1. Kawai T, Leventhal J, Madsen JC, Strober S, Turka LA, Wood KJ. Tolerance: one transplant for life. Transplantation. 2014; 98:117–121.
2. Nomura M, Yamashita K, Murakami M, Takehara M, Echizenya H, Sunahara M, et al. Induction of donor-specific tolerance by adenovirus-mediated CD40Ig gene therapy in rat liver transplantation. Transplantation. 2002; 73:1403–1410.
3. Oura T, Yamashita K, Suzuki T, Fukumori D, Watanabe M, Hirokata G, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant. 2012; 12:1740–1754.

4. Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012; 12:330–340.

5. Levitsky J. Operational tolerance: past lessons and future prospects. Liver Transpl. 2011; 17:222–232.

6. Shah JA, Navarro-Alvarez N, DeFazio M, Rosales IA, Elias N, Yeh H, et al. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann Surg. 2016; 263:1069–1071.
7. Oura T, Yamashita K, Suzuki T, Watanabe M, Hirokata G, Wakayama K, et al. A technique for orthotopic liver transplantation in cynomolgus monkeys. Transplantation. 2014; 98:e58–e60.

8. Spetzler VN, Goldaracena N, Knaak JM, Louis KS, Selzner N, Selzner M. Technique of porcine liver procurement and orthotopic transplantation using an active porto-caval shunt. J Vis Exp. 2015; (99):e52055.

9. Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation: a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014; 61:286–292.
11. Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014; 14:1599–1611.

12. Falcini F, Martini E, Marsili M, Benassai C, Fabbri LP, Tanini R, et al. Veno-venous bypass in experimental liver transplantation: portal-jugular versus cavalportal-jugular. G Chir. 1990; 11:206–210.
13. Lee KW, Cho CW, Lee JE, Park H, Choi GS, Jeong WK, et al. Morphologic and Doppler findings from hepatic ultrasonography of normal cynomolgus monkeys (Macaca fascicularis). J Am Assoc Lab Anim Sci. 2017; 56:177–180.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download