Abstract
Purpose
The aim of this study was to compare the long-term outcomes of total laparoscopic surgery with Natural Orifice Specimen Extraction (NOSE) with those for conventional laparoscopy (CL)-assisted surgery for treating rectal cancers.
Methods
We reviewed the prospectively collected records of 844 patients (163 NOSE and 681 CL) who underwent curative surgery for mid- or upper rectal cancers from January 2006 to November 2012. We applied propensity score analyses and compared oncological outcomes for the NOSE and CL groups in a 1:1 matched cohort.
Results
After propensity score matching, each group included 138 patients; the NOSE and CL groups did not differ significantly in terms of baseline clinical characteristics. The median follow-up was 57.7 months (interquartile range, 42.4–82.5 months). The combined 5-year local recurrence rate for all tumor stages was 4.1% (95% confidence interval [CI], 0.9%–7.4%) in the NOSE group and 3.0% (95% CI, 0%–6.3%) in the CL group (P = 0.355). The combined 5-year disease-free survival rates for all stages were 89.3% (95% CI, 84.3%–94.3%) in the NOSE group and 87.3% (95% CI, 81.8%–92.9%) in the CL group (P = 0.639). The postoperative mean fecal incontinence scores at 6, 12, and 24 months were similar between the 2 groups.
Many studies, including meta-analyses and multicenter prospective randomized clinical trials, have provided robust evidence that laparoscopic resection for rectal cancer is associated with a shorter length of hospital stay and better cosmetic results. Moreover, it has also been proven to be safe oncologically, thereby supporting its use as alternative to open surgery [123]. However, current conventional laparoscopic (CL)-assisted procedures usually require additional abdominal incisions for specimen extraction and constructing an anastomosis, and such minilaparotomy can often be the source of postoperative pain, surgical site infection, incisional hernia, and cosmetic problems [456].
Aimed at further reducing surgical trauma, a recent innovation—natural orifice transluminal endoscopic surgery (NOTES)—was developed [78]. Unfortunately, monumental technical hurdles have precluded widespread adoption of this technique. To overcome the problems with currently available endoscopic instruments, a stepwise clinical approach to pure NOTES which entails the use of hybrid procedures has been attempted. As a another refinement, the Natural Orifice Specimen Extraction (NOSE) has advantages over NOTES in that it maximizes the benefits of current multiport laparoscopic platform while using natural orifice as a intracorporeal anastomosis and specimen delivery route. NOSE can easily be incorporated to existing minimally invasive colorectal procedures without additional specialized instrumentation. During the last few years, various NOSE techniques have been described, with transrectal and transvaginal extractions being the most widely practiced [9101112].
We reported previously that the NOSE approach improved the short-term postoperative course in comparison with a CL approach even in radical surgery for colorectal malignancies [1314]. However, there remain concerns regarding the bacteriological and long-term oncological safety of such procedures. Therefore, the main objective of this study was to analyze the oncologic outcomes of NOSE compared with the CL procedure in patients with rectal cancers.
Consecutive cases of patients who underwent curative surgery for mid- or upper rectal cancer with the inferior margins located within 15 cm from the anal verge comprised the dataset for this retrospective analysis. In 2002, we initiated a prospective computerized database of patients with colorectal cancer [14]. Demographic characteristics, perioperative and pathology results, and follow-up data were recorded prospectively. Based on this surgical registry, we retrospectively identified a study population of 1,393 patients with rectal cancers between January 2006 and December 2012. We excluded 549 patients from the cohort for the following reasons: open surgery (n = 236); initial experiences with NOSE (n = 20); stage IV (n = 54); a history of other malignancies (n = 79); loss to follow-up within 12 months (n = 27); low rectal cancer with the inferior margins located within 4 cm of the anal verge (n = 133). The Institutional Review Board at Kyungpook National University Medical Center approved this study (KNUH-14-03). All patients gave their informed consent in writing prior to surgery during the study period.
The choice of surgical approach—NOSE or CL surgery—was determined by a joint preoperative decision between the patient and physician, and by intraoperative findings. In general, patients with a distant metastasis (M1) or tumors >5 cm in diameter on preoperative imaging were excluded from the NOSE procedure. The details of the CL technique and alternative approach (NOSE) were explained to all patients before surgery, and appropriate consent was obtained. Patients were also notified in advance that operation methods might be changed in response to unexpected intraoperative findings. We did not perform NOSE in certain intraoperative circumstance. Examples included some patients with peritoneal seeding not proven preoperatively, severe pelvic adhesions, the presence of grossly suspected T4 tumors, vaginal stenosis, or those with anal stenosis or incontinence.
Standard procedures including total mesorectal excision or tumor-specific mesorectal excision were performed in both groups. These included high ligature of the inferior mesenteric artery, medial-to-lateral mobilization of the sigmoid colon, complete splenic flexure takedown, and sharp pelvic dissection with a nerve-sparing technique. Although both types of surgery were performed uniformly through this step, different approaches were used in the NOSE group subsequently. We performed a complete intracorporeal resection and anastomosis of the bowel, with removal of the specimen through a transvaginal or transrectal route, as described previously [131516]. Briefly, in cases using transrectal NOSE, the proximal colon and the rectum distal to the tumor were divided by monopolar cauterization of the previous ligature (Fig. 1A). Then, a plastic bag was introduced through the anus, the specimen was collected, and the bag was removed through the anus (Fig. 1B). An anvil of a circular stapler was transferred through the rectal opening and placed at the end of the proximal colon. Finally, we completed an end-to-end anastomosis using intracorporeal purse-string sutures at both ends (Fig. 1C). For selected female patients, a posterior colpotomy was used as a route for transvaginal NOSE. A 60-mm endoscopic linear stapler was introduced through the vaginal port and a rectal transection was performed. A plastic bag was inserted through the port, and the specimen was placed. Next, the vaginal colpotomy was enlarged to 4–5 cm long to harvest the specimen. The proximal colon was exteriorized through the vagina to place the anvil (Fig. 1D). After that, a circular stapler was inserted into the rectal stump, and an end-to-end colorectal anastomosis was performed. Since 2008, we gradually applied the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) in the treatment of patients with rectal cancers. We preferred a hybrid robotic-laparoscopic approach, consisting of traditional laparoscopic technique for proximal lymphovascular dissection and colonic mobilization and the robotic system for rectal dissection. The same oncological principles and surgical steps were applied in both the laparoscopic and robotic procedures.
During the study period, fast-track program in rectal cancer surgery was not established. Generally, oral intake was allowed the day after the first flatus occurred. The patients were discharged after 2 or 3 days of tolerating a solid diet with no discomfort or complications. Postoperative complications were stratified by the Clavien-Dindo classification of surgical complications [17]. Anorectal function was mostly determined by the digital rectal exam and Jorge-Wexner score [18]. Questionnaires were administered in both groups at baseline, 3, 6, 12, and 24 months postoperatively.
Given that this was a retrospective cohort study and not a randomized trial, it was necessary to achieve comparability of the NOSE group and CL group with regard to potential confounding variables. Therefore, a propensity score matching method was used to match patients from each group in a 1:1 ratio. The propensity score was calculated by logistic regression analysis using the following covariates: sex, age, clinical T-stage, body mass index, preoperative chemoradiation status, and tumor height. Each patient was then matched 1:1 ratio using calipers of 0.04% width (20% of the standard deviation of the propensity score).
Before matching, baseline demographic and clinical characteristics were summarized using descriptive statistics. For continuous variables, data were presented as the mean ± standard deviation, and groups were compared using the unpaired t-test. The descriptive variables were analyzed by either chi-square analysis or Fisher exact test, as appropriate. Postmatching continuous variables are shown as mean ± standard deviation and categorical variables as absolute number (percentages). Statistical differences between the groups were tested with independent t-tests, and Mc Nemar tests.
The probabilities of local recurrence (LR) and of disease-free survival (DFS) were estimated using the Kaplan-Meier method and Cox proportional hazards model. The log-rank test was used to compare survival between groups and the minimum length of follow-up was 3 years. For backward conditional Cox proportional hazards analysis, variables were chosen by P < 0.05 in univariate analysis, along with age and sex. All statistical analyses were performed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) and Stata 13.0 (StataCorp LP., College Station, TX, USA).
After propensity score matching based on six previously described specific criteria, 138 patients subjected to NOSE were case matched with an equal number subjected to CL (Supplementary Fig 1). The NOSE and CL patient groups were balanced in terms of their demographics and baseline clinical characteristics (Table 1).
In the NOSE cohort, 60 and 78 of the interventions were conducted via transrectal and transvaginal access routes, respectively. Perioperative results and morbidity are summarized in Table 2. NOSE had a significantly longer operating time (difference in mean operating time = 22.1 minutes) than CL (P < 0.001). One patient in CL group required conversion to open surgery because of a difficult pelvic dissection. In the NOSE group, planned vaginal extraction was aborted in 2 patients because of the inadequate size of the posterior colpotomy. There were mismatches between large specimens and diameter of vaginal incision, and instead the specimens were extracted through a periumbilical minilaparotomy. A hybrid robotic-laparoscopic approach was performed for 5 patients (3.6%) in the CL group compared with 52 patients (37.7%) in the NOSE group (P < 0.001).
The overall incidence of surgical site infection was 0.7% (1 of 138) in the NOSE versus 5.8% (8 of 138) in the CL group (P = 0.039). Length of hospital stay was significantly shorter for patients going through NOSE surgery compared to CL surgery (9.8 days vs. 11.8 days, P = 0.007). Analysis of the pathologic data showed that histological differentiation, mean number of lymph node harvested, mean tumor size, and pathological TN stage were similar. Specifically, among midrectal cancer subset (n = 185), 65 patients in the NOSE group and 57 individuals in the CL groups were able to identify the circumferential resection margins (CRM) status from their pathological reports. The mean CRM were 6.9 ± 3.9 mm in the CL group and 7.8 ± 5.6 mm in the NOSE group, respectively (P = 242). The CRM was invaded (less than 1 mm) in two NOSE and three CL patients (1.4% vs. 2.2%, P = 0.685).
The median follow-up was 56.7 months (interquartile range, 47.1–84.9 months) for the NOSE group and 58.7 months (interquartile range, 47.0–80.8 months) for the CL group. The 3-year DFS for all tumor stages combined was 91.5% (95% confidence interval [CI], 87.1%–95.9%) in the NOSE group and 90.0% (95% CI, 85.2%–94.8%) in the CL group (P = 0.639 by log-rank test) (Fig. 2A). No significant differences in DFS were observed according to tumor stage (Table 3). There was also no difference in 3-year LR rates between the 2 groups (log-rank test, P = 0.355) (Fig. 2B). When patients were analyzed according to tumor stage, there were no differences in LR rates between the 2 groups (Table 3). Twenty-two patients in the NOSE group experienced tumor relapse (5 liver metastases, 6 lung metastases, 4 pelvic side-wall tumors, 3 in the perianastomotic area, and 4 others) compared with 19 in the CL group (4 liver metastases, 5 lung metastases, 6 pelvic side-wall tumors, 2 in the perianastomotic area, and 2 others). No recurrence was observed at the posterior colpotomy or transrectal access sites directly.
In univariate analysis of the prognostic factors affecting DFSs, NOSE and CL surgery had similar DFS (hazard ratio [HR], 0.794; 95% CI, 0.391–1.611; P = 0.523) (Table 4). With a multivariate analysis, positive lymphatic invasion (HR, 4.228; 95% CI, 1.927–9.277; P < 0.001), and positive lymph node metastasis (HR, 2.314; 95% CI, 0.964–5.555; P = 0.050) were independent prognostic factors affecting DFS.
Anal function was evaluated in 22 patients who underwent transrectal NOSE and 45 patients in the CL group. The comparisons of Wexner scores were similar in the 2 groups before surgery, and at 3, 6, 12, and 24 months after surgery (Fig. 3). At the 12th month, the mean score was 8.64 ± 4.2 in NOSE group and 7.39 ± 3.6 in CL group (P = 0.096) and at the 24th month, the mean score was 4.83 ± 3.8 in NOSE group and 3.77 ± 2.1 in CL group (P = 0.086). Two patients in each group still had a protective stoma at their last follow-up. Anastomotic strictures (1 case) and intractable chronic fistulas (3 cases) were causes of palliative or permanent stomas. During the study period, no patient requested a colostomy or secondary intervention because of anal incontinence after transrectal NOSE 11 female patients in the NOSE group who underwent transvaginal NOSE were sexually active at the time of surgery. All had resumed sexual activity by the follow-up survey, and none complained of dyspareunia or chronic vaginal discharges.
This retrospective, case-matched study showed that curative NOSE led to equivalent long-term oncological outcomes in patients with rectal cancers compared with conventional laparoscopy-assisted surgery. In addition, in our patients, the use of transrectal or transvaginal access routes for anastomosis and specimen extraction had no adverse effects on anorectal or sexual function during the midterm follow-up period after surgery. To our knowledge, this study that evaluated NOSE for patients with rectal cancers represents the largest series with the longest follow-up period ever published.
Avoidance of extraction site laparotomy is one of the most important features of NOSE. The presumed benefits of NOSE procedures over conventional minimal invasive approaches are less pain, faster recovery, better cosmetic results, and lower wound complication rates. The short-term outcomes after performing right colectomy using the transvaginal NOSE at the authors' institution have been published previously [14]. The results showed a mean operative time of 170.8 minutes with a significantly shorter hospital stay and less postoperative pain than CL surgery, as well as a high degree of patient satisfaction for the cosmetic outcome. To date, there have been 2 randomized clinical trials that compared the short-term operative outcomes in NOSE with CL colectomy [919]. They demonstrated that NOSE-based colectomy for left-sided colonic disease led to lower pain scores, fewer wound complications and better cosmetic outcomes. These are the most recent publications dealing with the surgical outcomes of NOSE, but are not the only ones. Other studies have evaluated the postoperative outcomes of NOSE for colorectal disease and have demonstrated its potential clinical benefits [1320212223].
One of the major concerns after using NOSE for rectal cancers is the fear of tumor cell implantation within the natural orifice site. There are no clear data about this type of tumor spillage. In our series, four patients in the NOSE group experienced LRs but none of those suffered from transrectal or transvaginal site tumor relapses. Currently, we routinely place the specimen in a protective bag before extraction or when delivering it through the anus or a posterior colpotomy site. In addition, we did not apply NOSE to those cases who were expected to have tumor compression because the tumor was bigger than the natural orifice, or who would have a risk of cancer cell implantation because there was serosal exposure to the tumor in intraoperative findings. With regard to specimen size and natural orifice characteristics, we do not have data showing any clear cutoff value above which the NOSE approach is contraindicated. However, the risk of tumor cell seeding during specimen delivery via a natural orifice would not be higher than that with transabdominal extraction, if the surgeon ensures proper protection of the specimen and selects cases carefully.
Apart from oncologic issues, there are some theoretical concerns over functional impairments with NOSE, such as anal sphincter injury or pelvic floor dysfunction. The vaginal route has probably the greatest literature coverage, and gynecologists have been using the vagina as a route for specimen extraction in various procedures for many years. Indeed, over the past decade several studies have reported vaginal extraction of a wide array of organs such as ovarian tumors, pancreas, kidney, or spleen with excellent results in terms of morbidity [2425262728]. In our study, changes in questionnaire responses were not significantly different between the 2 groups regarding anorectal function at 6, 12, or 24 months after surgery. In most cases, transrectal specimen extraction was conducted without any technical struggle because of the high elasticity of the anal sphincter muscle. Taken together, we consider that both the transrectal and transvaginal access routes have enough tissue elasticity and wound healing potential to harvest colorectal specimens.
In the literature, the precise definition of NOSE in patients with rectal cancers is controversial [29]. In our study, the definition of NOSE was “a specimen removed through an internal incision via the vagina or rectum, thus avoiding any minilaparotomy which has been conventionally necessary.” A broad definition could include intersphincteric resection with a coloanal anastomosis or a “down-to-up” transanal total mesorectal excision. The definitions and values used to measure postoperative results vary extensively and preclude an accurate comparison of oncologic or functional outcomes between studies. We considered that it would be difficult to distinguish between CL and NOSE when the cases available for intersphincteric resection or transanal extraction were included in our study. Accordingly, we excluded patients with low rectal cancers (i.e., <5 cm from the anal verge) to perform a more homogeneous cohort-comparison study based on our definition.
Although our validated matching model resulted in 2 study groups with quite similar propensity variables, this study was still limited by its retrospective nature. Hybrid robotic-laparoscopic procedures were performed more often in the NOSE than in the CL groups. This difference could be interpreted as reflecting the tendency of the surgeon trying to make the NOSE procedure easier by using the advantages of a robotic system. Indeed, we found that the robotic system was particularly useful for certain steps of NOSE, including the application of purse-string sutures to the distal rectal stump, transection of the distal end with a uniform distal margin, or intracorporeal closure of the posterior colpotomy incision. However, we believe that the impact of this selection bias was limited for our assessment of oncologic outcomes because the same standardized surgical principles were applied in both techniques. Insufficient data regarding functional outcomes was another limitation of this study. We only analyzed anorectal functions in 22 patients after transrectal NOSE and the objective data on gynecological disturbances following transvaginal NOSE were incomplete. The current focus of our research includes prospective evaluation of the quality of life, anal function, and genitourinary function following NOSE.
Our findings suggest that NOSE can be performed in selected patients with mid- and upper rectal cancers without compromising oncologic safety. NOSE is simpler than pure NOTES and ensures better short-term outcomes compared with conventional laparoscopy-assisted surgery. We anticipate that the NOSE procedure will play an important bridging role in moving current minimal invasive surgery toward purely “scarless” surgery.
Figures and Tables
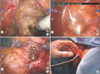 | Fig. 1A Natural Orifice Specimen Extraction (NOSE) approach for rectal cancer. (A) The rectum distal to the tumor was divided using monopolar cautery. (B) During transrectal NOSE, a plastic bag was introduced to remove the specimen through the anus. (C) Double purse-string sutures were applied after placement of the circular stapler. (D) The proximal colon was exteriorized through the vagina to place the anvil after transvaginal specimen extraction. |
 | Fig. 2Kaplan-Meier survival curve of disease-free survival (A) and local recurrence (B) in conventional laparoscopy-assisted surgery (CL) and Natural Orifice Specimen Extraction (NOSE) groups. |
 | Fig. 3Preoperative and postoperative follow-up scores after transrectal Natural Orifice Specimen Extraction (NOSE) and conventional laparoscopy-assisted surgery (CL). POD, postoperative day. |
Table 2
Perioperative outcomes

Values are presented as mean ± standard deviation or number (%).
NOSE, Natural Orifice Specimen Extraction; CL, conventional laparoscopy-assisted surgery.
a)Requiring a nasogastric drainage before discharge. b)Requiring reinsertion of the Foley catheter. c)Total number of patients suffered from postoperative complication more than 30 days after discharge.
References
1. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015; 372:1324–1332.

2. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014; 15:767–774.

3. Park JS, Choi GS, Jun SH, Park SY, Kim HJ. Long-term outcomes after laparoscopic surgery versus open surgery for rectal cancer: a propensity score analysis. Ann Surg Oncol. 2013; 20:2633–2640.

4. Singh R, Omiccioli A, Hegge S, McKinley C. Does the extraction-site location in laparoscopic colorectal surgery have an impact on incisional hernia rates? Surg Endosc. 2008; 22:2596–2600.

5. Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002; 16:1420–1425.

6. Romy S, Eisenring MC, Bettschart V, Petignat C, Francioli P, Troillet N. Laparoscope use and surgical site infections in digestive surgery. Ann Surg. 2008; 247:627–632.

7. Rolanda C, Lima E, Pego JM, Henriques-Coelho T, Silva D, Moreira I, et al. Third-generation cholecystectomy by natural orifices: transgastric and transvesical combined approach (with video). Gastrointest Endosc. 2007; 65:111–117.

8. Zorron R, Filgueiras M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A. NOTES. Transvaginal cholecystectomy: report of the first case. Surg Innov. 2007; 14:279–283.

9. Wolthuis AM, Fieuws S, Van Den Bosch A, de Buck van Overstraeten A, D'Hoore A. Randomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extraction. Br J Surg. 2015; 102:630–637.

10. Leroy J, Costantino F, Cahill RA, D'Agostino J, Morales A, Mutter D, et al. Laparoscopic resection with transanal specimen extraction for sigmoid diverticulitis. Br J Surg. 2011; 98:1327–1334.

11. Park JS, Choi GS, Lim KH, Jang YS, Kim HJ, Park SY, et al. Clinical outcome of laparoscopic right hemicolectomy with transvaginal resection, anastomosis, and retrieval of specimen. Dis Colon Rectum. 2010; 53:1473–1479.
12. Awad Z. Laparoscopic total colectomy with transvaginal extraction of the colon and ileorectal anastomosis. Ann Surg Oncol. 2014; 21:3029.

13. Melich G, Jeong DH, Hur H, Baik SH, Faria J, Kim NK, et al. Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy--analysis of learning curves for a novice minimally invasive surgeon. Can J Surg. 2014; 57:331–336.
14. Park JS, Choi GS, Kim HJ, Park SY, Jun SH. Natural orifice specimen extraction versus conventional laparoscopically assisted right hemicolectomy. Br J Surg. 2011; 98:710–715.

15. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010; 17:3195–3202.

16. Choi GS, Park IJ, Kang BM, Lim KH, Jun SH. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc. 2009; 23:2831–2835.

17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
18. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993; 36:77–97.

19. Leung AL, Cheung HY, Fok BK, Chung CC, Li MK, Tang CN. Prospective randomized trial of hybrid NOTES colectomy versus conventional laparoscopic colectomy for left-sided colonic tumors. World J Surg. 2013; 37:2678–2682.

20. Awad ZT, Griffin R. Laparoscopic right hemicolectomy: a comparison of natural orifice versus transabdominal specimen extraction. Surg Endosc. 2014; 28:2871–2876.

21. Xingmao Z, Haitao Z, Jianwei L, Huirong H, Junjie H, Zhixiang Z. Totally laparoscopic resection with natural orifice specimen extraction (NOSE) has more advantages comparing with laparoscopic-assisted resection for selected patients with sigmoid colon or rectal cancer. Int J Colorectal Dis. 2014; 29:1119–1124.

22. Ma B, Huang XZ, Gao P, Zhao JH, Song YX, Sun JX, et al. Laparoscopic resection with natural orifice specimen extraction versus conventional laparoscopy for colorectal disease: a meta-analysis. Int J Colorectal Dis. 2015; 30:1479–1488.

23. Costantino FA, Diana M, Wall J, Leroy J, Mutter D, Marescaux J. Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections. Surg Endosc. 2012; 26:1495–1500.

24. Uccella S, Cromi A, Bogani G, Casarin J, Serati M, Ghezzi F. Transvaginal specimen extraction at laparoscopy without concomitant hysterectomy: our experience and systematic review of the literature. J Minim Invasive Gynecol. 2013; 20:583–590.

25. Gill IS, Cherullo EE, Meraney AM, Borsuk F, Murphy DP, Falcone T. Vaginal extraction of the intact specimen following laparoscopic radical nephrectomy. J Urol. 2002; 167:238–241.

26. Mofid H, Emmermann A, Alm M, Zornig C. Transvaginal specimen removal after laparoscopic distal pancreatic resection. Langenbecks Arch Surg. 2013; 398:1001–1005.

27. Vereczkei A, Illenyi L, Arany A, Szabo Z, Toth L, Horváth OP. Transvaginal extraction of the laparoscopically removed spleen. Surg Endosc. 2003; 17:157.

28. Park YH, Kim KT, Bae JB, Kim HH. Transvaginal and transrectal natural orifice translumenal endoscopic surgery nephrectomy in a porcine survival model: comparison with conventional laparoscopic nephrectomy. J Endourol. 2015; 29:351–356.

29. D'Hoore A, Wolthuis AM. Laparoscopic low anterior resection and transanal pull-through for low rectal cancer: a Natural Orifice Specimen Extraction (NOSE) technique. Colorectal Dis. 2011; 13:Suppl 7. 28–31.
SUPPLEMENTARY MATERIAL
Supplementary Fig. 1 can be found via https://www.astr.or.kr/src/sm/astr-94-26-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download