Abstract
Purpose
The detection rate of brain metastasis (BM) from colorectal cancer (CRC) is increasing. This study was designed to analyze the clinical features of BM and prognosis according to the therapeutic modalities.
Methods
A total of 19 cases were collected in this study between November 2008 and December 2015. We reviewed the patients' demographic data and the clinical features of BM retrospectively and investigated their prognostic significance.
Results
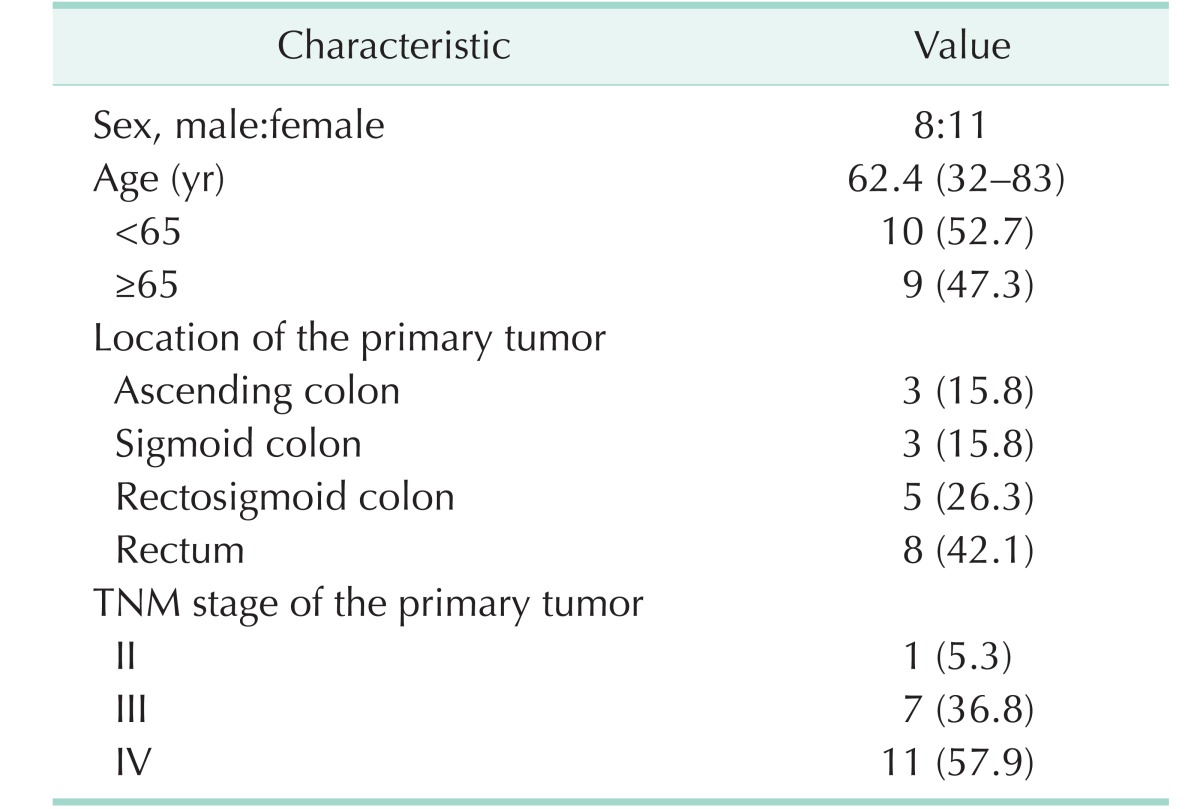
Nineteen patients included 8 male and 11 female patients. The median age at diagnosis of BM was 62.4 years (range, 32–83 years). The median interval between diagnosis of CRC and BM was 39 months (range, 0–98 months). Eighteen patients (94.7%) had extracranial metastasis at the diagnosis of BM. Lung was the most common site of extracranial metastasis in 14 patients (73.7%). Synchronous BMs were found at the diagnosis of primary CRC in 2 patients (10.5%). The location of primary CRC was the colon in 6 patients (31.6%) and the rectum in 13 patients (68.4%). At the diagnosis of BM, 10 patients (52.6%) had a solitary BM. The common neurologic symptoms were headache in 8 cases (42.1%) and ataxia in 6 cases (31.6%). The median survival after the diagnosis of BM was 3 months (range, 1–10 months). The patients who underwent surgery plus stereotactic radiosurgery (SRS) had an improved survival (range, 3–10 months) than the other patients (range, 1–6 months) (P = 0.016).
Colorectal cancer (CRC) is the third most common cancer, and the fourth leading cause of cancer-related deaths worldwide [12345]. With advances in the diagnostic radiographic techniques and treatment of CRC, survival of patients with CRC has improved over time and the incidence of brain metastasis (BM) from CRC has gradually increased as well [2356789]. Without treatment, the prognosis of patients with BM is typically poor with median survival of 1–2 months [258101112]. However, with active treatments including neurosurgical resection, whole brain radiotherapy (WBRT), and stereotactic radiosurgery (SRS), the prognosis of these patients can be improved [3571213].
BM from CRC is a late stage phenomenon; therefore, the patients who have BM may be predicted to have poorer survival. The incidence rate of BM is low and the patient survival is not long; therefore, it is not frequently studied in a single center. Therefore, the purpose of this study is to analyze the clinicopathologic features and to assess the prognostic factors of BM in CRC at a single center.
A retrospective analysis was made of 3,952 patients who were diagnosed with colorectal adenocarcinoma between November 2008 and December 2015. Nineteen cases were collected in 3,952 patients. The 19 patients were diagnosed with colorectal adenocarcinoma pathologically and BM by radiologic imaging. Their medical records were reviewed, including clinical characteristics of the primary tumor and BM, neurologic symptoms, location and number of BMs, dimension of BM, Karnofsky performance status (KPS), recursive partitioning analysis (RPA), presence of extracranial metastasis, treatment modalities for BM, and survival. Metachronous metastases were defined as the diagnosis of metastases 12 months after diagnosis of the primary tumor. Treatment modalities for BM included neurosurgical resection, SRS (Gamma-knife radiosurgery), and whole brain radiotherapy (WBRT). Decision regarding treatment for BM was made by experienced neurosurgeons.
The Karnofsky Performance Scale (KPS) is an evaluation tool for functional impairment. This can be used to estimate the patients' condition in order to endure the therapy and to predict the prognosis. KPS more than 70% is defined when the patient is able to carry on normal activity and to work; no special care needed [14].
RPA classification used in this study was profoundly related to the prognosis of patients with BM. Evaluation criteria were KPS, age, status of the primary tumor, and presence of extracranial metastasis. Based on these 4 criteria, patients were evaluated and classified into 3 prognostic classes. RPA class I consisted of patients with KPS of 70 and higher, aged 65 years or younger with controlled primary cancer and no extracranial metastasis. RPA class III included patients with KPS of less than 70%, and RPA class II consisted of patients who had not been qualified into class I or III [15].
Statistical analysis was using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Characteristics of BM, treatment strategy and survival were analyzed using the Kaplan-Meier method. Assessment of individual factors affecting survival was performed using the log-rank test. P-values < 0.05 were regarded statistically significant.
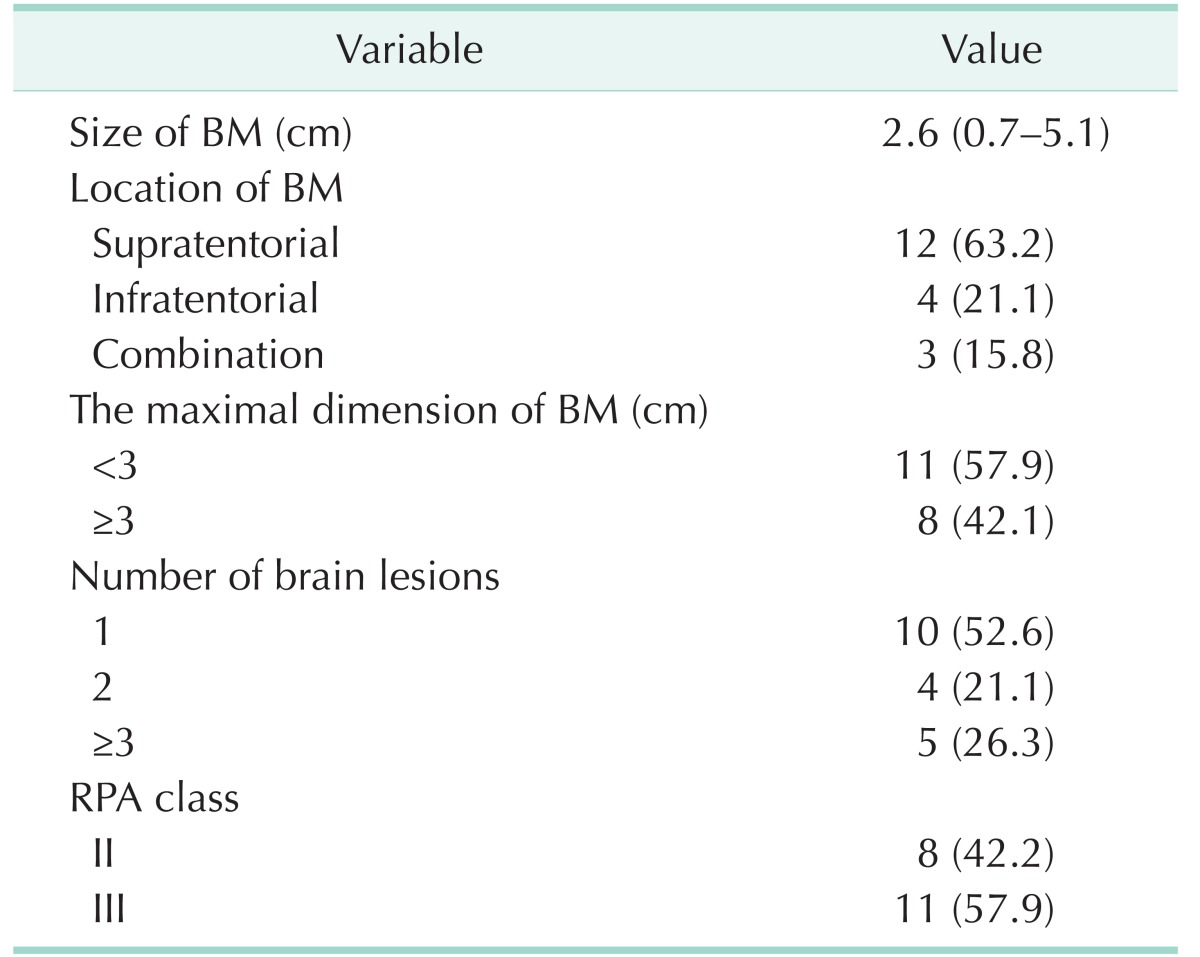
A total of 19 patients were enrolled, including 8 men and 11 women. Median age was 62.4 years (range, 32–83 years). The most common location of the primary tumor was the rectum in 8 cases and the rectosigmoid colon in 5 cases. TNM stage of the primary tumor was stage IV in 11 cases (57.9 %) and stage III in 7 cases (36.8 %). Characteristics of patients are shown in Tables 1, 2.
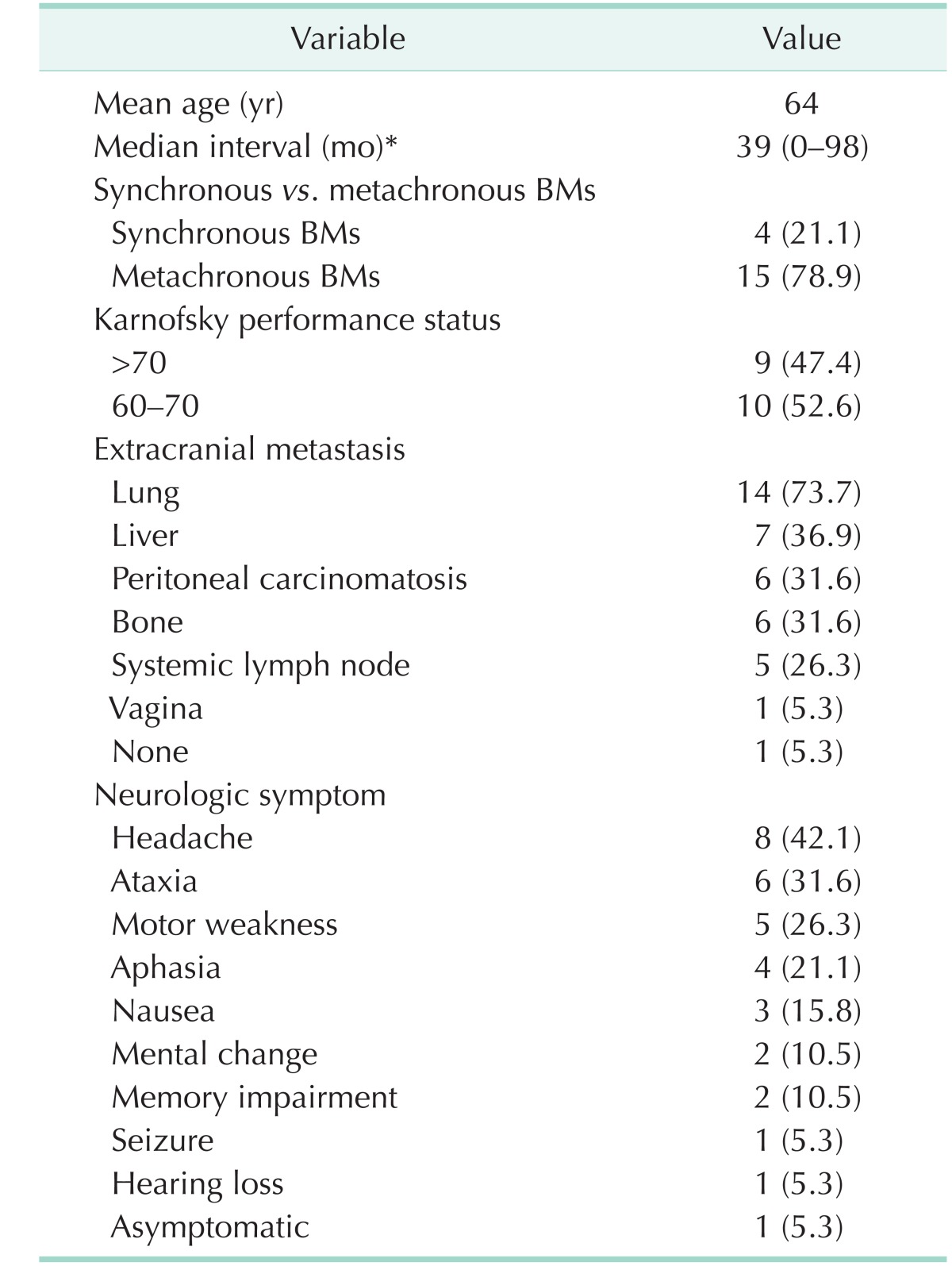
Four patients had simultaneous incident BMs, and 7 patients had metachronous BMs. The clinical features of patients with BM are demonstrated in Table 3. The median interval between the diagnosis of primary CRC and the diagnosis of BM was 39 months (range, 0–98 months). Synchronous BMs were found in 4 cases (21.1%) and metachronous BMs were found in 15 cases (78.9%). KPS more than 70% was found in 9 cases.
At the time of BM diagnosis, extracranial metastasis was found in 18 cases (94.7%). Lung was the most common site of extracranial metastasis in 14 cases (73.7%).
Most patients had multiple symptoms. The most common neurologic symptom was headache in 8 cases (42.1%). Other symptoms were ataxia, motor weakness, aphasia, nausea, mental change, and memory impairment. The clinical features of BM varied, as shown in Table 4.
Mean size of BM was 2.6 cm (range, 0.7–5.1 cm). BM with maximal dimension less than 3 cm was found in 11 cases (57.9%). Supratentorial BM was found in 12 cases (63.2%), infratentorial BM was found in 4 cases, and both supra and infratentorial BMs was found in 3 cases. The number of metastasis was 1 in 10 cases, 2 in 4 cases, and more than 3 in 5 cases. According to the RPA classification, 8 patients were classified as class II and 11 patients were classified as class III.
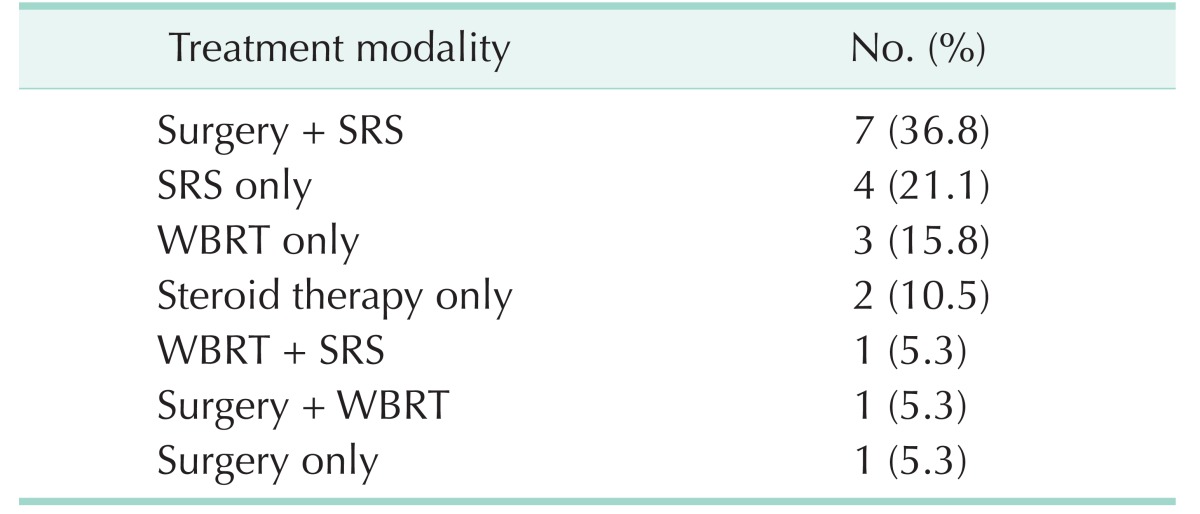
Patients with BM received the following therapies: Four patients were treated with SRS alone, 3 patients were treated with WBRT alone, and 1 patient was treated with surgery (craniotomy and tumor removal) alone. Two patients received steroid therapy alone for pain relief without undergoing any treatment. Seven patients underwent combined therapy of surgery and SRS. Five patients received SRS after surgery for recurrent BM and 2 patients were simultaneously treated with surgery and SRS (Table 5).
The median survival was 3 months after the diagnosis of BM (range, 1–10 months). The median survival of patients who received surgery plus SRS was 5 months (range, 3–10 months). SRS alone-treated patients had a median survival of 2.5 months (range, 1–5 months). WBRT alone-treated patients had a median survival of 3 months (range, 2–4 months). The patients who received combined therapy of surgery plus SRS showed a statistical significance in terms of prognosis than the other patients (P = 0.016).
In this study, the incidence of BM from CRC was 0.76%. The patients who were treated with active treatment including surgical removal and stereotactic surgery showed better oncologic survival.
The incidence of BM in CRC is relatively low compared to that of metastasis to other organs [245610111617]. Little is known about the clinical features at the time of presentation of BM, and hence, a proper guideline has not yet been established [24578101819]. Therefore, we investigated the clinical features related to BM through clinical analysis and the appropriate treatment policy for patients.
The incidence of BM has been reported to vary from about 0.3%–5.0% [25819]. A definite pattern of BM could not be found in many studies [17]. However, the number of patients with BM is increasing with the increase in the incidence of CRC [2]. Also, another reason for the increase in BM is that the oncologic outcome is improved with the development of the surgical technique and adjuvant chemotherapy in CRC [6]. The development of diagnostic techniques related to brain imaging has increased the accuracy of diagnosis of small brain lesions [691120].
It has been reported that the location of the primary tumor; the colon or rectum, is related to the incidence of BM [234]. The result of our study was similar, and it showed that the patients with rectal cancer have a higher tendency for BM. This is caused due to the same reason that pulmonary metastasis is more common in rectal cancer than in colon cancer [341113]. In our analysis of extracranial metastasis, lung metastasis was more frequent than metastasis to other organs. These clinical characteristics occur due to the differences in venous drainage from the rectum. It is well known that the middle and lower rectal veins in the rectum enter the internal iliac vein and have a circulatory system through the inferior vena cava. The mechanism associated with the development of BM through the circulatory venous system is described in other studies [31116]. In addition, Mongan et al. have also described the mechanism of BM in the form of tumor emboli through systemic circulation of the venous plexus [421].
Time interval between the diagnosis of primary CRC and the diagnosis of BM was reported to be about 36 months (range, 21–51 months), and our result was similar [578101113]. In many literatures, most BMs after colon cancer surgeries show late manifestations [5618]. This is because the blood-brain barrier (BBB) is formed by continuous tight junctions, which include endothelial cells, astroglia, and pericytes, resulting in a structurally solid blocking effect [6]. Moreover, the development of the surgical technique for CRC and anticancer therapy for systemic control seem to cause an increase in the survival rate and late manifestation [3]. However, when BM occurs, the tight junctions between endothelial cells become loosened, and tumor cells become highly permeable to enter the brain. Even though the chances for tumor cells to enter the cerebral parenchyma increase, the molecular formulation of anticancer therapy for systemic control is too large to pass the BBB. Therefore, brain becomes a kind of store-house for metastatic tumor cells.
Although the molecular mechanism of tumor cells that cross the BBB has not been fully established; it is reported that CXCR4, a receptor of the chemokine CXCL12, induces instability of blood vessels and it can increase the permeability of brain endothelial cells. Colorectal carcinoma cells show higher levels of CXCR4 than normal colonic epithelial cells [6]. It could suggest the relationship between CXCR4 and BM [46].
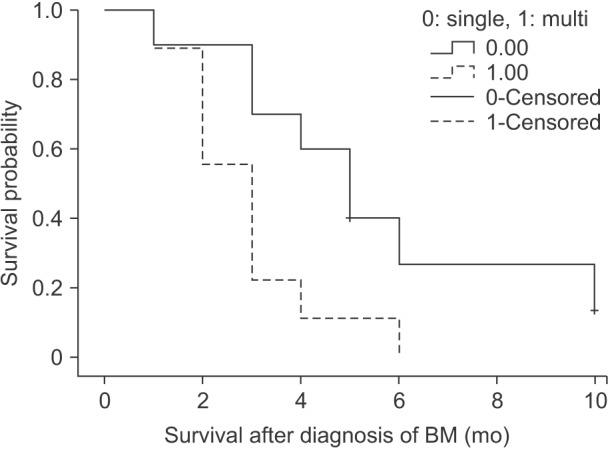
In other studies, the mean survival period according to the treatment modality for BM has been reported to be about 15 months for surgical resection, 6–10 months for stereotactic radiotherapy, and 4–6 months for whole brain irradiation [313]. Besides monotherapy, combined therapy of surgical resection and radiotherapy has been reported to provide a significantly better survival rate [1622]. Because of better survival after combined therapy including surgical resection, it is important that BM becomes a target for surgical resection. The possibility of surgical treatment for BM depends on the number of BM lesions and location of BM [378]. In our study, the number of BM lesions was found to be an independent factor affecting prognosis of patients, and this finding is similar to that in previous studies [5812131823]. However, for deciding about resection of BM, one more thing that should be considered other than the resectability of BM is general performance status [310]. Therefore, there might be some kind of a bias that the patients in whom resectability of BM was possible and who had good general performance were selected for surgical treatment for BM, and they showed better survival.
The main neurologic symptom of BM was reported to be gait disturbance, but our patients most commonly suffered from headache, which is nonspecific [34]. Therefore, we considered performing active evaluation of BM when the patients complain of nonspecific neurologic symptoms, regardless of the period from surgical treatment for CRC.
However, it is presumed that the incidence of BM can be estimated to be higher in all patients of CRC because of the patients may be asymptomatic or cause of death may be another metastatic organs.
In conclusion, these efforts make it possible to detect BM earlier and to perform surgical resection for BM. Furthermore, this could be helpful for improving the survival of patients with BM.
References
1. Pan J, Xin L, Ma YF, Hu LH, Li ZS. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am J Gastroenterol. 2016; 111:355–365. PMID: 26753884.

2. Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer--Incidence and patient characteristics. BMC Cancer. 2016; 16:260. PMID: 27037031.

3. Gu XD, Cai YT, Zhou YM, Li ZY, Xiang JB, Chen ZY. Prognostic factors and multidisciplinary treatment modalities for brain metastases from colorectal cancer: analysis of 93 patients. BMC Cancer. 2015; 15:902. PMID: 26572484.

4. Mongan JP, Fadul CE, Cole BF, Zaki BI, Suriawinata AA, Ripple GH, et al. Brain metastases from colorectal cancer: risk factors, incidence, and the possible role of chemokines. Clin Colorectal Cancer. 2009; 8:100–105.

5. Jiang XB, Yang QY, Sai K, Zhang XH, Chen ZP, Mou YG. Brain metastases from colorectal carcinoma: a description of 60 cases in a single Chinese cancer center. Tumour Biol. 2011; 32:1249–1256. PMID: 21913132.

6. Zang YW, Gu XD, Xiang JB, Chen ZY. Brain metastases from colorectal cancer: microenvironment and molecular mechanisms. Int J Mol Sci. 2012; 13:15784–15800. PMID: 23443093.

7. Suzuki Y, Yamaguchi T, Matsumoto H, Nakano D, Honda G, Shinoura N, et al. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. 2014; 57:56–63. PMID: 24316946.

8. Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, et al. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. 2012; 106:144–148. PMID: 22287384.

9. Tan WS, Ho KS, Eu KW. Brain metastases in colorectal cancers. World J Surg. 2009; 33:817–821. PMID: 19194739.

10. Michl M, Thurmaier J, Schubert-Fritschle G, Wiedemann M, Laubender RP, Nüssler NC, et al. Brain metastasis in colorectal cancer patients: survival and analysis of prognostic factors. Clin Colorectal Cancer. 2015; 14:281–290. PMID: 26123495.

11. Tanriverdi O, Kaytan-Saglam E, Ulger S, Bayoglu IV, Turker I, Ozturk-Topcu T, et al. The clinical and pathological features of 133 colorectal cancer patients with brain metastasis: a multicenter retrospective analysis of the Gastrointestinal Tumors Working Committee of the Turkish Oncology Group (TOG). Med Oncol. 2014; 31:152. PMID: 25108599.

12. Kim HJ, Huh JW, Jung TY, Kim IY, Kim HR, Jung S, et al. Clinical outcome with gamma-knife surgery or surgery for brain metastases from colorectal cancer. J Clin Neurosci. 2013; 20:1417–1421. PMID: 23910824.

13. Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis. 2012; 14:e378–e385. PMID: 22288509.

14. Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004; 10:3345–3348. PMID: 15484315.

15. Caroli M, Di Cristofori A, Lucarella F, Raneri FA, Portaluri F, Gaini SM. Surgical brain metastases: management and outcome related to prognostic indexes: a critical review of a ten-year series. ISRN Surg. 2011; 2011:207103. PMID: 22084749.

16. Damiens K, Ayoub JP, Lemieux B, Aubin F, Saliba W, Campeau MP, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012; 19:254–258. PMID: 23144573.

17. Magni E, Santoro L, Ravenda PS, Leonardi MC, Bonomo G, Monfardini L, et al. Brain metastases from colorectal cancer: main clinical factors conditioning outcome. Int J Colorectal Dis. 2014; 29:201–208. PMID: 24158623.

18. Kruser TJ, Chao ST, Elson P, Barnett GH, Vogelbaum MA, Angelov L, et al. Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer. 2008; 113:158–165. PMID: 18459179.
19. Tevlin R, Larkin JO, Hyland JM, O'Connell PR, Winter DC. Brain metastasis from colorectal carcinoma: a single cancer centre experience. Ir J Med Sci. 2015; 184:673–675. PMID: 25802245.

20. Mege D, Ouaissi M, Fuks D, Metellus P, Peltier J, Dufour H, et al. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res. 2013; 33:5645–5648. PMID: 24324111.
21. Onodera H, Nagayama S, Tachibana T, Fujimoto A, Imamura M. Brain metastasis from colorectal cancer. Int J Colorectal Dis. 2005; 20:57–61. PMID: 15309466.

22. Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology. 2009; 76:369–374. PMID: 19321946.

23. Tokoro T, Okuno K, Hida JC, Ueda K, Yoshifuji T, Daito K, et al. Prognostic factors for patients with advanced colorectal cancer and symptomatic brain metastases. Clin Colorectal Cancer. 2014; 13:226–231. PMID: 25442813.

Fig. 1
Kaplan-Meier survival curves for survival after the diagnosis of brain metastasis (BM) according to the treatment(P = 0.005).
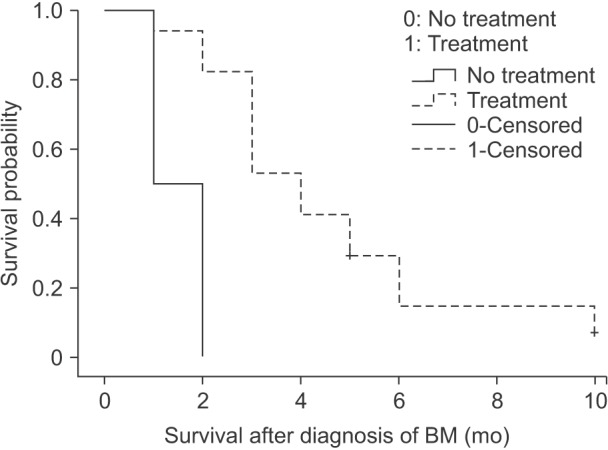
Fig. 2
Kaplan-Meier survival curves for survival after the diagnosis of brain metastasis (BM) according to the number of BMs (P = 0.027).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download