Abstract
Purpose
Neurogenic myositis ossificans (NMO) in patients with traumatic spinal cord or brain injuries can cause severe joint ankylosis or compromise neurovascularture. The purpose of this study was to evaluate the clinical and radiological outcomes of and review considerations relevant to surgical resection of NMO of the hip joint.
Materials and Methods
Six patients (9 hips) underwent periarticular NMO resection between 2015 and 2017. The medical records of these patients were retrospectively reviewed. Preoperative computed tomography including angiography was performed to determine osteoma location and size. Improvement in hip motion allowing sitting was considered the sole indicator of a successful surgery. The anterior approach was used in all patients. The ranges of motion (ROM) before and after surgery were compared.
Results
The mean time from accident to surgery was 3.6 years. Average ROM improved from 24.3°(flexion and extension) to 98.5°(flexion and extension) after surgery, and improvement was maintained at the last follow-up. No commom complications (e.g., deep infection, severe hematoma, deep vein thrombosis) occurred in any patient. Improvement in ROM in one hip in which surgical resection was performed 10 years after the accident was not satisfactory owing to the pathologic changes in the joint.
Neurogenic myositis ossificans (NMO) is a pathologic condition characterized by formation of heterotopic periarticular ossification after severe brain or spinal cord injury1). The reported incidence of NMO ranges from 10% to 23% after spinal cord injury, and large joints, often hips, are prone to be affected234). In cases of minor ossification, conservative treatment might suffice to facilitate performing daily life activities without discomfort at an early or even later stage of ossification4).
However, in cases of large ossifications leading to severe ankylosis or neurovascular compromise, surgical excision of heterotopic bone formations may be the only effective option5). Surgery requires rigorous organization and planning owing to the high risk of vascular injury, and the decision to attempt surgery must be made after providing precise counseling to the patient before surgery and highlighting the expected level of functional gain6).
To the best of our knowledge, no case series of NMO of the hip joint requiring surgical resection have been reported in East Asia. This study aimed to evaluate the clinical and radiological outcomes of and review the challenges in surgical resection of NMO of the hip joint.
Medical records of 6 patients (9 hips; all men), who underwent surgical resection of NMO between 2015 and 2017 were retrospectively reviewed. Five patients had a history of spinal cord injury and 1 experienced a brain injury. The indication for surgery in all patients was severe periarticular heterotopic ossification limiting functional activity, particularly range of motion (ROM). The patients in our study experienced extreme difficulty in maintaining body balance and could not sit on a wheelchair. We decided on surgical resection after the maturity of the NMO was confirmed radiologically when a normal serum alkaline phosphatase level was maintained for more than 6 months. The average age of patients at the time of surgery was 40 years; the mean follow-up period was 20 months (Table 1).
Preoperative computed tomography (CT) scans including angiographies were performed for all patients to determine the location and size of osteomas, and the volume, morphology, borders, and changes in hip joint; its relationship to the joint capsule and femoral and circumflex vessels was also evaluated. Physical examinations including ROM and neurological examination to determine the severity of cord injury were performed at the time of admission. Ambulatory status was graded using the Gross Motor Function Classification System (GMFCS) score7).
In 3 patients who had undergone surgery in both hips, staged surgery (1 week between the first and the following contralateral surgery) was performed, given the extensive blood loss during surgery. In all patients, 1 g of ferric carboxymaltose (Ferinject®; Vifor Pharma, Bern, Switzerland) was injected before surgery, and the same dose was administered after surgery along with 1 g of tranexamic acid to minimize the risk of bleeding.
The anterior approach was used for resection of the heterotopic ossification (HO) in all patients and an anterior soft-tissue release was performed if necessary8). The NMO affected bone was surrounded by a periosteum-like capsule and lay between muscular planes (Fig. 1A). The surgical margins were determined using the line parallel to the iliac crest on the upper border and the lesser trochanter as the lower-upper border. All bone blocks that hindered hip activity were removed, and subsequently, manipulation of the hip was performed intraoperatively to evaluate the ROM of the hip and potential bone impingement (Fig. 1B). Physiotherapy was provided after removal of drains after surgery, and indometacin (100 mg) once daily for 6 weeks was administered to all patients to prevent recurrence9). After discharge, patients were routinely followed up at 6 weeks, 3 months, 6 months, and 12 months postoperatively and annually thereafter. Follow-up examinations focused on ROM and any recurrent ossification.
We reviewed all surgical reports and collected the following data: age, sex, date of the accident and type of accident (brain trauma, spinal cord trauma, level of cord injury or other), post-accident ambulatory function (using GMFCS)10), pre- and post-operative ROM, surgery time, and surgical complications.
Descriptive statistics were reported as means and standard deviations for continuous variables and as total number and percentage for discrete variables. The Wilcoxon signed rank test was used to compare preoperative ROM to ROM immediately after surgery and that at the last follow-up. Statistical analyses were performed using SPSS for Windows version 16.0 (SPSS, Chicago, IL, USA). The study design and protocol of this work were approved by the institutional review board of Inje University Seoul Paik Hospital (PAIK-2018-02-005).
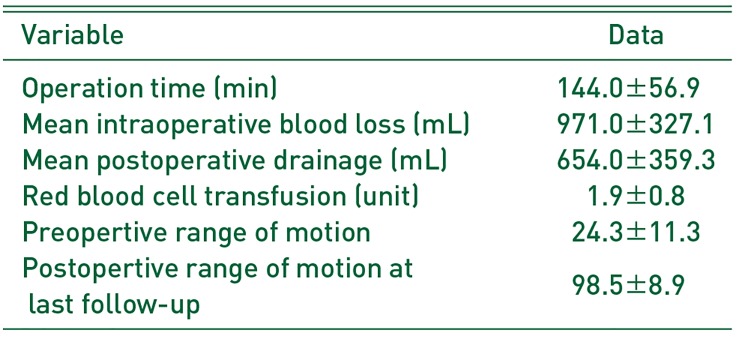
Mean operation time was 144 minutes, and no intraoperative iatrogenic fracture of the femur or the acetabulum occurred, even in patients with severe ankylosis (Fig. 1C). All patients showed remarkable increase in ROM after surgery and during the follow-up period (Table 2). The average ROM improved from 24.3°(flexion and extension) preoperatively to 98.5°postoperatively (flexion and extension), with a maximum improvement at last follow-up of 98.5°(p<0.01). Complications (e.g., deep infection, severe hematoma, deep vein thrombosis) were not experienced by any patient. Of the 9 hip joints, 2 redeveloped small bone islands in the soft tissues 6 months after surgery; however, no severe ossification recurrence was observed in any patient and ROM improvement immediately after surgery was maintained (Fig. 1D). For two hips (hip number 5 and 6) for which resection was performed 10 years after the accident, ROM improvement was not satisfactory owing to pathologic changes in the joint (Fig. 2).
The pathophysiology of NMO is not completely understood. However, several groups have provided clear evidence in support of a humoral mechanism for accelerated fracture healing leading to increased bone formation111213). Serum taken from patients with an injured central nervous system contains increased circulating growth factors (e.g., insulin-like growth factor II, platelet-derived growth factor, interleukin-1, and interleukin-6)141516). More recently, specific neurotransmitters (e.g., leptin) have also been shown to play a potential role in bone metabolism via the hypothalamus and sympathetic nervous system1718). However, not all patients with similar characteristics and demographics develop NMO indicating that several genes might be responsible for the development of NMO19).
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) for prophylaxis of NMO is based on mechanisms involving the systemic inhibition of prostaglandins, as evidenced by circulating humoral factors20). Low-dose radiation therapy is another option; however, we only use NSAIDs based on the patient's preference. During follow-up, recurrence was found in 2 patients in the form of a small bony lesion which was not clinically significant.
NMO is relatively different from HO induced by surgery. NMO develops in connective tissue between muscular planes without involving the muscle121). On the other hand, HO has been shown to destroy surrounding muscles22). Quadriceps femoris and iliopsoas muscles are known to be the most affected muscles in NMO23). Intraoperatively, we found that the sartorius, quadriceps, and iliopsoas muscles which lay anterior to the hip were intact but slightly atrophied because of the large bone mass. No femoral neurovascular structures were entrapped in the NMO in our patients and it was easy to retract the femoral neurovascular bundle from NMO.
Although there is a lack of consensus regarding the appropriate timing for excision of ossification, prolonged delay can lead to poor functional recovery because of pathologic changes induced by prolonged immobilization24). Stover et al.25) observed joint surface narrowing with atrophy and severe osteoarthritic change and suggested that prolonged immobilization stimulates fibro-fatty proliferation and fibrous ankylosis. In our series, 1 patient underwent resection 10 years after the accident; severe osteoarthritic changes were observed, which resulted in unsatisfactory postoperative ROM (80°). Although acetabular dysplasia has been noted after surgical resection, pressure necrosis at the cartilage-cartilage interface could result from a prolonged ankylosing period26).
The patient's conditions (e.g., pain, swelling, a high serum alkaline phosphatase level, 3-phase bone scan, CT) have been suggested as indicators of the maturity of ossification2). We performed surgery when CT indicated lesions with smooth, well-demarcated margins and defined trabeculations and a normal serum alkaline phosphatase level was maintained for more than 6 months27). Many authors advocate the use of bone scan to evaluate the maturity of NMO14). However, we were able to perform pre-operative bones scans only in some patients owing to patients' condition.
Our study had some limitations. First, it was a retrospective study including a small number of patients. Second, the follow-up period varied among patients. Third, clinical results other than the improvement of ROM were not evaluated. However, most of the patients in our cohort were quadriplegic who hardly felt pain and could not walk.
Surgical treatment of HO of the hip is effective when performed in patients with appropriate indications. Preoperative CT is essential for assessing joint space change and preoperative planning. Prolonged delay in surgery can lead to pathological intra-articular changes, which can affect functional recovery.
References
1. Sakellariou VI, Grigoriou E, Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification following traumatic brain injury and spinal cord injury: insight into the etiology and pathophysiology. J Musculoskelet Neuronal Interact. 2012; 12:230–240. PMID: 23196266.
2. van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002; 40:313–326. PMID: 12080459.

3. Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991; (263):13–29. PMID: 1899635.

4. Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res. 1988; (233):86–101.

5. Genet F, Marmorat JL, Lautridou C, Schnitzler A, Mailhan L, Denormandie P. Impact of late surgical intervention on heterotopic ossification of the hip after traumatic neurological injury. J Bone Joint Surg Br. 2009; 91:1493–1498. PMID: 19880896.

6. Denormandie P, de l'Escalopier N, Gatin L, Grelier A, Genêt F. Resection of neurogenic heterotopic ossification (NHO) of the hip. Orthop Traumatol Surg Res. 2018; 104:S121–S127. PMID: 29174871.

7. Mayson TA, Ward V, Davies KR, et al. Reliability of retrospective assignment of gross motor function classification system scores. Dev Neurorehabil. 2013; 16:207–209. PMID: 23323825.

8. Garland DE. Surgical approaches for resection of heterotopic ossification in traumatic brain-injured adults. Clin Orthop Relat Res. 1991; (263):59–70. PMID: 1899638.

9. Baird EO, Kang QK. Prophylaxis of heterotopic ossification - an updated review. J Orthop Surg Res. 2009; 4:12. PMID: 19379483.

10. McCormick A, Brien M, Plourde J, Wood E, Rosenbaum P, McLean J. Stability of the Gross Motor Function Classification System in adults with cerebral palsy. Dev Med Child Neurol. 2007; 49:265–269. PMID: 17376136.

11. Bidner SM, Rubins IM, Desjardins JV, Zukor DJ, Goltzman D. Evidence for a humoral mechanism for enhanced osteogenesis after head injury. J Bone Joint Surg Am. 1990; 72:1144–1149. PMID: 2398084.

12. Gautschi OP, Toffoli AM, Joesbury KA, Skirving AP, Filgueira L, Zellweger R. Osteoinductive effect of cerebrospinal fluid from brain-injured patients. J Neurotrauma. 2007; 24:154–162. PMID: 17263679.

13. Kantak AP, Shah NN. Extensive surgical wound lavage reduces the incidence and severity of heterotopic ossification in primary total hip replacement: a study of 175 hip replacements. Hip Pelvis. 2017; 29:234–239. PMID: 29250497.

14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002; 43:346–353. PMID: 11884494.
15. Hata K, Takahata Y, Murakami T, Nishimura R. Transcriptional network controlling endochondral ossification. J Bone Metab. 2017; 24:75–82. PMID: 28642850.

16. Kim JS, Yoon SS, Kim YH, Ryu JS. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke. 1996; 27:1553–1557. PMID: 8784129.
17. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002; 111:305–317. PMID: 12419242.

18. Wang L, Tang X, Zhang H, Yuan J, Ding H, Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med. 2011; 34:501–509. PMID: 22118258.

19. Mitchell EJ, Canter J, Norris P, Jenkins J, Morris J. The genetics of heterotopic ossification: insight into the bone remodeling pathway. J Orthop Trauma. 2010; 24:530–533. PMID: 20736788.

20. Banovac K, Williams JM, Patrick LD, Levi A. Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib). Spinal Cord. 2004; 42:707–710. PMID: 15179440.

21. Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005; 37:129–136. PMID: 16040468.

22. Wu XB, Yang MH, Zhu SW, et al. Surgical resection of severe heterotopic ossification after open reduction and internal fixation of acetabular fractures: a case series of 18 patients. Injury. 2014; 45:1604–1610. PMID: 24917211.

23. Wick L, Berger M, Knecht H, Glücker T, Ledermann HP. Magnetic resonance signal alterations in the acute onset of heterotopic ossification in patients with spinal cord injury. Eur Radiol. 2005; 15:1867–1875. PMID: 15856244.

24. Campbell TM, Reilly K, Laneuville O, Uhthoff H, Trudel G. Bone replaces articular cartilage in the rat knee joint after prolonged immobilization. Bone. 2018; 106:42–51. PMID: 28974461.

25. Stover SL, Niemann KM, Tulloss JR. Experience with surgical resection of heterotopic bone in spinal cord injury patients. Clin Orthop Relat Res. 1991; (263):71–77.

26. Nomura M, Sakitani N, Iwasawa H, et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthritis Cartilage. 2017; 25:727–736. PMID: 27916560.

27. Law-Ye B, Hangard C, Felter A, et al. Pre-surgical CT-assessment of neurogenic myositis ossificans of the hip and risk factors of recurrence: a series of 101 consecutive patients. BMC Musculoskelet Disord. 2016; 17:433. PMID: 27756329.
Fig. 1
An 18-year-old male patient complaining of severe ankylosis in the right hip joint. (A) Intra-operative image of the right hip showing an irregularly shaped, 13 cm wide, 25 cm long osteoma. (B) The osteomas were very large and had to be split into several pieces to be removed. (C) Anteroposterior radiogram showing complete ankylosis of the hip. (D) Radiogram at 1 year after surgery showed small calcific deposit but no evidence of recurrence of severe ossification.
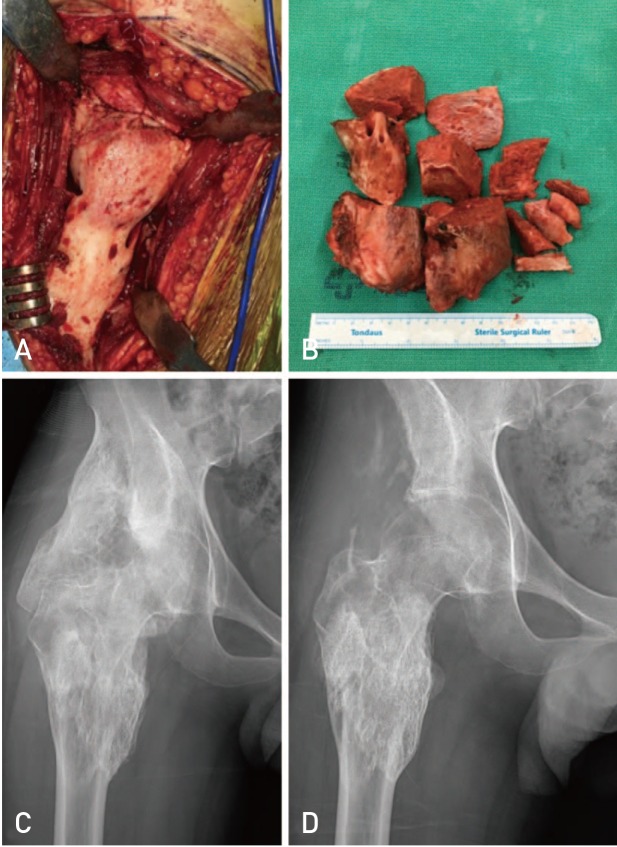
Fig. 2
Anteroposterior radiogram of a 31-year-old male patient reveals large bilateral osteomas of the hips in a bridge from the iliac bone to the femoral shaft (A). (B) Postoperative radiogram 6 months after surgical resection of neurogenic myositis ossificans showing joint destruction with collapsed joint space.
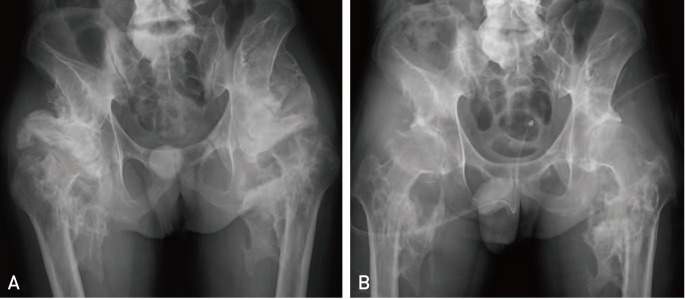
Table 1
Preoperative Demographic Details of Patients
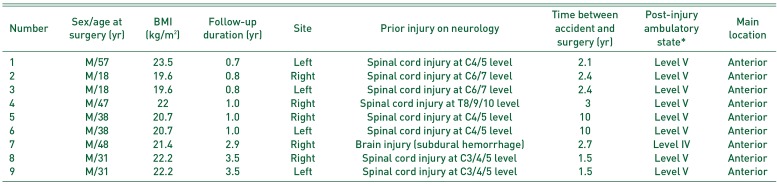
Table 2
Operation Details in Patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download