Abstract
The causes of osteolytic lesions found in radiological examinations are not quite certain. Therefore, to determine the appropriate treatment method, various approaches and analyzes are required to find the real cause. Hyperparathyroidism is one of the diseases which forms osteolytic bone lesions so-called brown tumor. A 55-year-old woman who had painful osteolytic bone lesions in both hip joint areas was diagnosed as parathyroid carcinoma after serial work-up. She underwent parathyroidectomy and follow-up imaging showed a decrease in brown tumor size and bone consolidation in the subchondral bone destruction area. Proper evaluation of osteolytic bone lesions helps to avoid unnecessary operative treatments and the first choice for the treatment of osteolytic bone lesions caused by parathyroid carcinoma is parathyroidectomy.
There are various causes of osteolytic bone lesions, and once one is detected on a plain radiograph, a proper approach to diagnosing the source is required. One potential cause of focal reactive osteolytic lesions—also known as brown tumors—is hyperparathyroidism1). The incidence of osteolytic brown tumors is 3% and 2% in patients with primary and secondary hyperparathyroidism, respectively2).
Most cases of hyperparathyroidism are caused by a solitary parathyroid adenoma (80–85%), and the remaining 15–20% are associated with hyperplasia or multiple adenomas. The incidence of parathyroid carcinoma is very rare (i.e., less than 1%)3). Hyperparathyroidism promotes excessive osteoclast activity, and the increased resorption leads to cortical bone destruction and fibrous cyst formation with hemosiderin deposition4).
Our case of parathyroid carcinoma presented with clinical symptoms of multiple osteolytic lesions in both hip joints. A diagnosis of parathyroid carcinoma (primary tumor) was made following evaluation of the apparent bone metastasis. This is a rare report of a case involving recovery of an osteolytic lesion involving the supra-acetabular area following parathyroidectomy and treatment with vitamin D and a calcium substitute.
Each author certifies that his institution has approved the reporting of this case report and that all investigations were conducted in conformity with ethical principles of research. The patient was informed that data from the case would be submitted for publication, and gave their consent.
A 55-year-old female experienced acute left hip pain without a history of trauma 1 week prior to our initial evaluation. The patient also complained of transient right hip pain which had commenced 6 months prior. With the exception of a laparoscopic myomectomy due to a cystic mass in the uterus performed 23 years prior to her initial evaluation, there was no indication of an underlying disease. Her physical examination was normal, except for a painful hip joint range of motion limitation during flexion of the right hip.
An initial plain radiograph showed a 33.09×42.24 mm-sized osteolytic lesion with an incomplete linear pathological neck fracture in the left femur, and a 48.52×52.03 mm-sized mixed osteolytic and osteoblastic lesion in the right pelvis, just above the acetabulum (Fig. 1A). After thorough evaluation of her general condition, surgery was planned. The lumbar bone mineral density was evaluated using dual X-ray absorptiometry and revealed severe osteoporosis (0.606 g/cm2; T score, −4.2 standard deviation). To evaluate the cortical bone state of the osteolytic lesion, three-dimensional reconstruction computed tomography (CT) was performed. Cortical disruption of the right acetabulum, iliac bone, and lateral cortex of the left femoral neck was observed (Fig. 2A, B).
Admission laboratory tests showed abnormal total calcium levels (15.8 mg/dL; normal, 8.4–10.2 mg/dL) and ionized calcium (4.46 mEq/L; normal, 2.2–2.6 mEq/L). Additional laboratory tests (e.g., endocrinology and tumor markers) were conducted to aid in differential diagnosis of the bone lesion. Carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) were both in the normal range (CEA: 1.09 ng/mL; normal, 0–5 ng/mL and CA 19–9: 9.93 U/mL; normal, 0–39 U/mL); however, a mild elevation in β2-microglobulin was observed (3,292.4 µg/L; normal, 970–2,640 µg/L). To better quantify the severity of osteoporosis, an endocrinology test was conducted and revealed low levels of vitamin D (25(OH)D) (7.2 ng/mL; normal, 30–50 ng/mL). For further evaluation, hip magnetic resonance imaging (MRI) and positron emission tomography CT (PET-CT) of torso were performed. On MRI, demineralization of the osteolytic lesion and a septate cystic mass were observed (Fig. 3). Torso PET-CT revealed a primary lesion showing a 3-cm hypermetabolic mass in the right thyroid lobe (Fig. 4). Neck ultrasonography showed a 4-cm hypoechoic nodule, and fine-needle aspiration was subsequently performed, leading to a suspicions of follicular neoplasm. Bone metabolism markers, including serum parathyroid hormone (PTH), phosphorus, and alkaline phosphatase were as follows: 2,646.40 pg/mL (normal, 15.0–68.3 pg/mL), 2.0 mg/dL (normal, 2.5–5.5 mg/dL), 711 U/L (normal, 35–129 U/L) (Table 1).
Based on the image and laboratory findings, a diagnosis of a brown tumor caused by primary hyperparathyroidism was made. The patient underwent parathyroidectomy by an otolaryngologist one week after the first visit to our hospital. A hard fixed 15×40 mm-sized mass was resected, and histopathology confirmed parathyroid carcinoma. Because of the high risk of pathological fracture in the osteolytic bone lesion of the left proximal femur, prophylactic compression hip screw fixation was performed two weeks after parathyroidectomy (Fig. 1B). During surgery, a bone biopsy of the left proximal femoral neck lesion and lateral side cortical ballooning area were conducted leading to the diagnosis of a brown tumor. With respect to the bone lesion in the right supra-acetabular area, we planned non weight-bearing and conservative treatment. We explained to the patient that she may need a joint replacement if the pain did not resolve and the bone destruction continued.
Vitamin D and calcium medication were maintained after surgery, and weight bearing was allowed 3 months after the operation. The right hip joint area pain subsided by the 4 month follow up visit, and plain radiograph revealed bone formation in the right acetabular dome (Fig. 1C). One year after the operation, the patient had no problems during ambulation, and a plain radiograph and CT scan showed more bone consolidation (Fig. 2C, D). Moreover, markers of bone metabolism were normal and medication was discontinued.
Brown tumors are benign osteolytic lesions of the bone, usually due to primary or secondary hyperparathyroidism. Most brown tumors occur as solitary lesions, but in some rare cases present as multiple lesions5,6). Commonly affected bones are the pelvis, ribs, femurs, humerus, and other long bones7). Brown tumors present well-defined borders in plain radiographs and CT scans. Subperiosteal resorption in cortical bones and cystic lesions are the findings. Upon MRI, heterogeneous hypointense and isointense muscle can be observed on T1-weighted images8).
Massive bone resorption is caused by hyperactivity of osteoclasts and osteoblasts, resulting in bone replacement with fibrous tissues. Oversecretion of parathyroid hormone causes systemic decalcification of bone lesions.
The primary treatment approach for hyperparathyroidismrelated brown tumors (with the goal of decreasing the tumor's size) is surgery9); previous studies report improvement in bone density and recovery without further treatment following this approach10). Surgical treatment of bone lesions is only performed in cases with a high risk of fracture. However, cases of bone destruction in the joint are not well-reported, especially in hip joints, which require a high biomechanical load.
Although clinical features and laboratory tests in our case revealed a high probability of a brown tumor, pathological evaluation was needed to rule out other malignant tumors. Histopathological findings of brown tumors are: i) an increased number of osteoclasts, ii) cyst formation, and iii) deposits of hemosiderin.
Accordingly, parathyroidectomy was performed as the primary treatment in our case, and follow-up imaging showed a reduction in brown tumor size and bone consolidation in the joint destruction area.
After parathyroid hormone levels normalized, conservative treatment with calcium and vitamin D was sufficient to resolve the large brown tumor in the joint area, even in the hip joint with continuous stress.
This case study is evidence of the value of proper preoperative evaluation of parathyroid carcinoma causing multiple osteolytic brown tumors, thus avoiding unnecessary operative treatment of symptomatic osteolytic lesions. Moreover, following parathyroidectomy—which is the standard of care for treating parathyroid carcinoma—a rapid decrease in serum calcium levels can occur requiring a high dose of replacement calcium. Therefore, the results of this case suggest that a proper period of medication and non-weight bearing can restore osteolytic subchondral bone lesion in supra-acetabular area without surgical intervention.
Figures and Tables
Fig. 1
Plain radiographs of the hip. (A) Large tumor lesion in the right acetabulum area involving the hip joint and the left femoral neck area. (B) Immediate postoperative image of prophylactic compressive hip screw fixation for a left femoral neck lesion. (C) The tumor gradually diminished in size 1 year after parathyroidectomy, with the appearance of calcification.

Fig. 2
Pelvic computed tomography showing (A) a large osteolytic lesion in the right pelvis with a thin cortex, shown in the axial view; and (B) hip joint area involvement, shown in the coronal view. (C) The tumor gradually diminished in size 1 year after parathyroidectomy, together with the appearance of calcification, shown in the axial view; and (D) subchondral bone regeneration involved in the right hip joint area, shown in the coronal view.
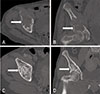
Fig. 3
Brown tumor lesion of the right acetabulum area, shown in magnetic resonance imaging (A) axial, (B) coronal T1-weighted image, and (C) coronal T2-weighted image.
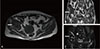
Fig. 4
(A) Fludeoxyglucose F 18 (FDG) positron emission tomography/computed tomography showing a 3.0-cm paratracheal lesion located at the posterior aspect of the right thyroid gland. The lesion demonstrates a low FDG-avid tumor (maximal standardized uptake value [SUVmax], 1.9). (B) Osteolytic and destructive lesions located in the right acetabulum area, suspected of being skeletal metastases with a SUVmax of 7.1.
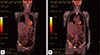
Table 1
Patient's Laboratory Test Results

References
1. Takeshita T, Takeshita K, Abe S, Takami H, Imamura T, Furui S. Brown tumor with fluid-fluid levels in a patient with primary hyperparathyroidism: radiological findings. Radiat Med. 2006; 24:631–634.

2. Hong WS, Sung MS, Chun KA, et al. Emphasis on the MR imaging findings of brown tumor: a report of five cases. Skeletal Radiol. 2011; 40:205–213.

3. Kebebew E, Clark OH. Parathyroid adenoma, hyperplasia, and carcinoma: localization, technical details of primary neck exploration, and treatment of hypercalcemic crisis. Surg Oncol Clin N Am. 1998; 7:721–748.
4. Andersen KF, Albrecht-Beste E. Brown tumors due to primary hyperparathyroidism in a patient with parathyroid carcinoma mimicking skeletal metastases on (18)F-FDG PET/CT. Diagnostics (Basel). 2015; 5:290–293.

5. Pumar JM, Alvarez M, Perez-Batallon A, Vidal J, Lado J, Bollar A. Brown tumor in secondary hyperparathyroidism, causing progressive paraplegia. Neuroradiology. 1990; 32:343.

6. Brown TW, Genant HK, Hattner RS, Orloff S, Potter DE. Multiple brown tumors in a patient with chronic renal failure and secondary hyperparathyroidism. AJR Am J Roentgenol. 1977; 128:131–134.

7. Jouan A, Zabraniecki L, Vincent V, Poix E, Fournié B. An unusual presentation of primary hyperparathyroidism: severe hypercalcemia and multiple brown tumors. Joint Bone Spine. 2008; 75:209–211.

8. Vernace N, Bancroft LW. Multiple brown tumors and pathologic patellar fracture in a patient with secondary hyperparathyroidism. Orthopedics. 2014; 37:564–567.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download