Abstract
Purpose
To investigate the incidence and risk factors of cystoid macular edema (CME) after silicone oil (SO) injection for retinal detachment.
Methods
Fifty-eight patients with retinal detachment treated by vitrectomy with SO tamponade during 2011 to 2015 were retrospectively assigned to CME and non-CME groups. Patients underwent complete ophthalmological examination, including color fundus photography and preoperative and postoperative optical coherence tomography. Risk factors for CME during SO tamponade were determined by regression analyses.
Results
Of the 58 eyes, 21 (36.2%) exhibited CME. The presence of posterior staphyloma in the CME group was significantly more frequent than in the non-CME group (p = 0.026). There were no significant differences in other demographic or clinical characteristics between the CME and non-CME groups. Significant correlations were observed between CME after vitrectomy with SO tamponade and the presence of posterior staphyloma (odds ratio, 4.03; p = 0.031). Of the 21 eyes with CME, 13 underwent SO removal, among which 11 experienced resolution of CME with or without further intervention.
Silicone oil (SO) is an effective endotamponade agent used in cases that require a long-term or permanent filling effect for the retention of anatomical reattachment of the retina [1234]. It is typically used in vitreoretinal surgery for treatment of retinal detachment (RD) with proliferative vitreoretinopathy (PVR), giant retinal tears, severe proliferative diabetic retinopathy, macular hole, uveitis, or trauma. However, SO injection is associated with a variety of complications in the anterior and posterior segments of the eye, including formation of intraconjunctival oil inclusion cysts, band keratopathy, cataract, glaucoma, and chronic hypotony [56789].
Macular edema results from either serous exudation from intraretinal capillaries between the outer plexiform and inner nuclear layers of the retina or swelling of the retinal Müller cells. Cystoid macular edema (CME) is defined as the localized expansion of the extracellular space in the macular area of the retina and is characterized by a radial cystic pattern in the perifoveal region [10]. Spectral-domain optical coherence tomography (SD-OCT) can provide high-resolution in vivo cross-sectional images of the retina even in the presence of intraocular SO [11] and can reveal histological changes in the retina. This imaging modality has been used recently to establish CME as one of the retinal complications of SO tamponade.
The purpose of this study was to examine the incidence and risk factors of CME after vitrectomy with SO tamponade for RD.
The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Severance Hospital, Seoul, Korea (4-2016-0637). Written informed consents were obtained. Medical charts of 93 consecutive patients (93 eyes) who were followed-up after vitrectomy with SO tamponade for treatment of RD between November 2012 and December 2015 were reviewed. All patients had received pars plana vitrectomy and SO injection for complicated RD associated with uveitis, giant retinal tears, subretinal hemorrhage due to polypoidal choroidal vasculopathy, or PVR with tractional RD. The exclusion criteria were as follows: diagnosis of tractional RD with proliferative diabetic retinopathy, diagnosis of CME by SD-OCT prior to surgery, unknown medical or surgical history, unavailability of OCT images acquired during SO tamponade, and follow-up loss during SO tamponade.
Data on demographic and clinical characteristics including age, sex, ophthalmological and medical history, visual acuity, and intraocular pressure (IOP) were acquired from medical charts. Intraoperative data included information on the cause of RD, macular on/off status, and history of combined cataract surgery or scleral buckling based on the surgical report. Postoperative data included information on visual acuity and IOP after SO tamponade, duration of SO tamponade, postoperative complications, lens status during SO tamponade, and OCT data acquired during SO tamponade.
Best-corrected visual acuity was measured using a Snellen chart and was converted to logarithm of the minimum angle resolution visual acuity for analysis. Diagnosis of CME was based on postoperative SD-OCT findings (Heidelberg Spectralis SD-OCT; Heidelberg Engineering, Heidelberg, Germany) of retinal thickening with the loss of the foveal pit with intraretinal cystoid spaces. Postoperative SD-OCT images were used to establish the presence or absence of posterior staphyloma, which was defined by an outpouching of the ocular wall with a curvature radius smaller than that of the surrounding ocular wall. The time of CME onset was defined as the time of its initial detection on postoperative OCT images. Duration of SO tamponade was defined as the interval between injection of SO and its removal or exchange. In patients who did not receive SO removal, duration of SO tamponade was defined as the interval between SO injection and final OCT scanning.
A single surgeon (SSK) performed all surgeries with the patient under general or local anesthesia induced by subtenon injection. Sclerotomies were placed 3.5 and 4.0 mm from the limbus in pseudophakic and phakic eyes, respectively. Following core vitrectomy, SO tamponade was performed after internal drainage and endolaser photocoagulation in eyes with rhegmatogenous RD with large retinal tears, hemorrhagic RD, or serous RD. Injection of SO was performed after the removal of the tractional membrane from the retina with tractional RD. For SO tamponade, Oxane SO (1,300 centistokes; Bausch & Lomb, Rochester, NY, USA) was used.
Comparison of categorical and distributed variables between the CME and non-CME groups was performed by Student's t-test and chi-square test. Risk factors for CME after SO tamponade were identified by univariate and multivariate conditional logistic regression analyses. We tried to gather statistically significant variables only via the Enter method of logistic regression analysis. Then, using selected variables, we applied the forward condition method to eliminate insignificant variables, because the method automatically extracts variables based on the level of the statistical significance. Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Values of p < 0.05 were considered to indicate statistical significance.
Of the 93 patients (93 eyes) who received SO injections, 35 were excluded for the following reasons: diagnosis of tractional RD with proliferative diabetic retinopathy (n = 21), follow-up loss during SO tamponade (n = 9), lack of OCT data acquired during SO tamponade (n = 2), uncertain medical history (n = 2), and diagnosis of CME by OCT before surgery for RD (n = 1). Thus, 58 eyes of 58 patients who had undergone vitrectomy with SO tamponade were finally enrolled.
Of the 58 eyes, 21 were diagnosed with CME by SD-OCT and assigned to the CME group. The incidence of CME after vitrectomy with SO tamponade was 36.2%. Median patient age in the CME and non-CME groups was 51.48 and 50.27 years, respectively (p = 0.826). Median duration of SO tamponade in the CME and non-CME groups was 276 and 273 days, respectively (p = 0.966). There were no significant differences between the two groups in terms of sex, laterality, medical history of hypertension or diabetes mellitus, preoperative best-corrected visual acuity, or IOP. Table 1 summarizes the demographic and clinical characteristics of the two groups.
Table 2 summarizes the clinical characteristics of RD and the treatment history of the two groups. The presence of posterior staphyloma in the CME group was significantly higher than in the non-CME group (p = 0.026). There were no significant differences between the two groups in the cause of RD, axial length, macular on/off status, surgical history, history of combined surgery, or lens status during SO tamponade.
Table 3 summarizes the results of univariate logistic regression analyses of risk factors for CME after vitrectomy with SO tamponade. The presence of posterior staphyloma (odds ratio, 4.03 [1.14 to 14.28]; p = 0.031) was significantly associated with CME.
Of the 21 eyes in the CME group, 8 did not receive SO removal or exchange immediately after SO tamponade. The remaining 13 eyes received SO removal, of which 11 experienced resolution of CME after the procedure (Fig. 1), while 2 did not exhibit any recovery (Fig. 2). Of the 11 eyes that recovered, 1 exhibited complete resolution of CME after posterior subtenon triamcinolone injection (PSTI) and 1 after intravitreal Avastin injection; CME in the remaining 9 eyes subsided spontaneously without further intervention. The average recovery time was 70 days (range, 6 to 287 days).
In the present study, the incidence of CME after vitrectomy with SO tamponade was found to be 36.2%, which is higher than that reported by previous studies (13.6% to 27.5%) [11121314]. Upon comparative evaluation of preoperative and postoperative SD-OCT images of 46 eyes that received vitrectomy with SO tamponade, Bae et al. [12] reported CME in 9 (19.6%) eyes before SO removal. Kiss et al. [13] reported the development of CME after SO tamponade only in eyes with RD caused by PVR (7 / 39 eyes, 17.1%). Rashad et al. [11] reported a 27.5% incidence of CME (14 of 51 eyes) using SD-OCT–based diagnosis. Karahan et al. [14] reported a 13.6% incidence of CME (3 of 22 eyes) among patients with rhegmatogenous RD who received SO tamponade. In these previous studies, SO was removed at earlier time points (3.6 to 9 months postoperatively) than in our study. It is likely that the incidence of CME was higher in this study than in other studies because of a longer presence of SO. On average, CME occurred on postoperative day 171 in our study (not shown), while SO was removed before this time point in most previous studies. In order to reduce the incidence of CME, it would be better to remove the SO as soon as possible.
In the present study, of the 13 eyes that received SO removal, 11 experienced resolution of CME; 2 eyes exhibited recovery after anti-vascular endothelial growth factor therapy or PSTI, while 9 exhibited spontaneous recovery. In the study by Bae et al. [12], 8 of 9 eyes recovered from CME within 6 months after surgery, regardless of the procedure for peeling of the internal limiting membrane during SO removal. In the study by Karahan et al. [14], CME subsided in all 3 eyes within 1 month after SO removal. Upon evaluation of 12 eyes, Lo et al. [15] reported spontaneous resolution of CME within 9 to 12 months post-surgery in all 4 eyes with increased retinal thickness. In these previous studies, the time of recovery from CME ranged from 1 to 12 months after SO removal without any treatment. In the present study, the majority of eyes that did not receive any treatment exhibited spontaneous resolution of CME within approximately 2 months. In two patients with persistent CME, the final OCT evaluation was performed only 286 and 293 days after SO removal. Since complete resolution of CME requires a maximum of 12 months, these two patients are expected to recover through anti-vascular endothelial growth factor therapy or PSTI.
In this study, the presence of posterior staphyloma (odds ratio, 4.03) was determined to be a risk factor for CME. SO tends to form a sphere due to its surface tension. Therefore, the greater is the sphericity of the vitreous cavity, the easier it would be for SO to fill the cavity completely. It is likely that a retro-oil space is formed at the site of the posterior staphyloma. The development of CME has been attributed to elevated levels of inflammatory factors, such as interleukin 6, as well as growth factors in retro-SO fluid between the SO and the retina [1617]. In our study, CME subsided spontaneously in 9 eyes; as in the previous studies, CME resolved only after SO removal without any intervention. These results support the hypothesis that SO removal helps resolve CME by redistribution of inflammatory factors into the vitreous cavity of the eye [1819].
The presence of posterior staphyloma and axial length are known to be risk factors for the development of myopia [20]. However, in our study, the presence of posterior staphyloma was found to be a risk factor for CME development, whereas axial length was not. Staphyloma can occur in eyes without long axial lengths. Curtin [20] showed that, in eyes with type I staphyloma—which is the most common type—axial length ranged from 25 to 38 mm. Therefore, Curtin [20] pointed out that axial length is not a reliable marker to define pathologic myopia and concluded that pathologic myopia should be defined by the presence of staphyloma. Wang et al. [21] recently reported clinical features of staphylomas in eyes with axial lengths less than 26.5 mm. Thus, eye wall outpouching without long axial length should also be considered as posterior staphyloma. Therefore, it seems reasonable to conclude that the shape of the eyeball relative to the area of the retro-SO space is more important in the development of CME than the degree of myopia.
There have not been many studies regarding the risk factors of the development of macular edema related to SO tamponade status. Azzolini et al. [22] reported that macular off status and longer SO permanence affect the incidence of ME associated with long-term SO tamponade. Scheerlinck et al. [23] also reported that the duration of SO tamponade was a statistically significant factor related to the incidence of unexplained visual loss. In contrast, in the present study, macular on/off status and duration of SO tamponade were determined to have an insignificant effect on the incidence of ME. Further studies are needed on this subject.
This study has a few limitations, mostly because of its retrospective nature. The actual development of CME and its resolution might be faster than those determined in the present study, because OCT images were not acquired at every outpatient visit. Further prospective studies involving larger cohorts are required to confirm our findings.
In conclusion, the present study indicates that the incidence of CME after vitrectomy with SO tamponade is as high as 36.2% in patients with RD. Additionally, the presence of posterior staphyloma might be a risk factor for CME. Most eyes that underwent SO removal experienced spontaneous resolution of CME. In patients with RD with posterior staphyloma, the potential risk of CME due to SO injection should be evaluated carefully by SD-OCT during SO tamponade.
Figures and Tables
Fig. 1
Rhegmatogenous retinal detachment due to retinal hole in the left eye of a 75-year-old female patient. The patient had no history of hypertension or diabetes mellitus and had received cataract surgery in both eyes. Preoperative visual acuity and intraocular pressure were 0.02 Snellen units and 13 mmHg, respectively. The patient received combined vitrectomy with scleral encircling and additional laser treatment. Cystoid macular edema was detected on spectral-domain optical coherence tomography images acquired 237 days after surgery. (A) Oil removal and posterior subtenon triamcinolone injection were performed 251 days after silicone oil injection. (B) Remission of cystoid macular edema was observed on spectral-domain optical coherence tomography images acquired 1 month later.
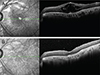
Fig. 2
Rhegmatogenous retinal detachment due to retinal hole in the left eye of a 23-year-old female patient. The patient had no history of hypertension or diabetes mellitus. Preoperative visual acuity and intraocular pressure were 0.05 Snellen units and 23 mmHg, respectively. The patient received combined vitrectomy with scleral encircling and additional laser treatment. Cystoid macular edema was detected on spectral-domain optical coherence tomography images acquired 218 days after surgery. (A) Oil removal was performed 343 days after silicone oil injection. (B) However, remaining cystoid macular edema was observed on spectral-domain optical coherence tomography images acquired 293 days after oil removal.
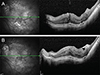
Table 2
Comparison of clinical characteristics between patients with and without cystoid macular edema

References
1. Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 1. Arch Ophthalmol. 1992; 110:770–779.
2. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 2. Arch Ophthalmol. 1992; 110:780–792.
3. Laqua H, Lucke K, Foerster MH. Development and current status of silicone oil surgery. Klin Monbl Augenheilkd. 1988; 192:277–283.
4. Riedel KG, Gabel VP, Neubauer L, et al. Intravitreal silicone oil injection: complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol. 1990; 228:19–23.

5. Casswell AG, Gregor ZJ. Silicone oil removal I The effect on the complications of silicone oil. Br J Ophthalmol. 1987; 71:893–897.

6. Casswell AG, Gregor ZJ. Silicone oil removal II Operative and postoperative complications. Br J Ophthalmol. 1987; 71:898–902.

7. Franks WA, Leaver PK. Removal of silicone oil: rewards and penalties. Eye (Lond). 1991; 5(Pt 3):333–337.
8. Hutton WL, Azen SP, Blumenkranz MS, et al. The effects of silicone oil removal. Silicone Study Report 6. Arch Ophthalmol. 1994; 112:778–785.
9. La Heij EC, Hendrikse F, Kessels AG. Results and complications of temporary silicone oil tamponade in patients with complicated retinal detachments. Retina. 2001; 21:107–114.

10. Colin J. The role of NSAIDs in the management of postoperative ophthalmic inflammation. Drugs. 2007; 67:1291–1308.

11. Rashad MA, Mohamed AA, Ahmed AI. Value of optical coherence tomography in the detection of macular pathology before the removal of silicone oil. Clin Ophthalmol. 2016; 10:121–135.

12. Bae SH, Hwang JS, Yu HG. Comparative analysis of macular microstructure by spectral-domain optical coherence tomography before and after silicone oil removal. Retina. 2012; 32:1874–1883.

13. Kiss CG, Richter-Muksch S, Sacu S, et al. Anatomy and function of the macula after surgery for retinal detachment complicated by proliferative vitreoretinopathy. Am J Ophthalmol. 2007; 144:872–877.

14. Karahan E, Tuncer I, Zengin MO, et al. Spontaneous resolution of macular edema after silicone oil removal. Int J Ophthalmol. 2014; 7:1005–1009.
15. Lo DM, Flaxel CJ, Fawzi AA. Macular effects of silicone oil tamponade: optical coherence tomography findings during and after silicone oil removal. Curr Eye Res. 2017; 42:98–103.

16. Christensen UC, la Cour M. Visual loss after use of intraocular silicone oil associated with thinning of inner retinal layers. Acta Ophthalmol. 2012; 90:733–737.

17. Wolf S, Schon V, Meier P, Wiedemann P. Silicone oil-RMN3 mixture (“heavy silicone oil”) as internal tamponade for complicated retinal detachment. Retina. 2003; 23:335–342.

18. Asaria RH, Kon CH, Bunce C, et al. Silicone oil concentrates fibrogenic growth factors in the retro-oil fluid. Br J Ophthalmol. 2004; 88:1439–1442.

19. Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009; 116:73–79.

20. Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1977; 75:67–86.
21. Wang NK, Wu YM, Wang JP, et al. Clinical characteristics of posterior staphylomas in myopic eyes with axial length shorter than 26.5 millimeters. Am J Ophthalmol. 2016; 162:180–190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download