INTRODUCTION
MATERIALS AND METHODS
Participants
Treatments
Definitions, assessments and follow ups
Statistics
RESULTS
Baseline characteristics
Table 1
Baseline Characteristics
IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count; HLA, human leukocyte antigen; TNC, total nuclear cell.
Data are presented as means±standard deviations, medians (interquartile range), or counts (percentages). Comparison between two groups were conducted by t test, Wilcoxon rank sum test, or chi-square test. p<0.05 was considered significant.
Clinical efficacy by UCBI+IST and IST treatment
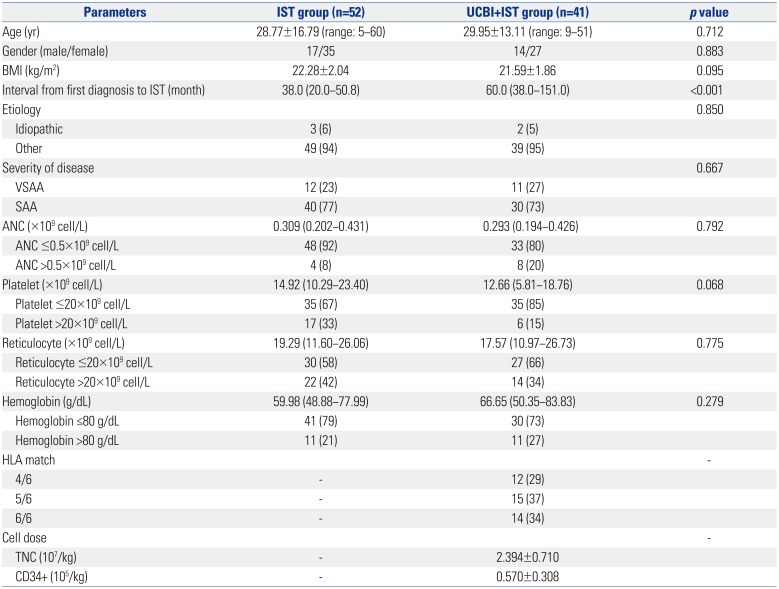
Fig. 1
Comparison of treatment responses between UCBI+IST and IST treatments at 6 months. Both CR and ORR were higher in the UCBI+IST group than the IST group. Differences between groups were detected by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ORR, overall response rate; CR, complete response; PR, partial response; NR, no remission.
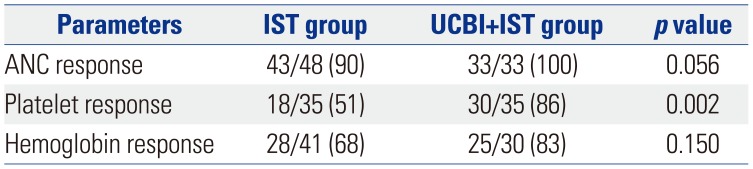
Hematopoietic recovery rate in UCBI+IST and IST groups
Time to hematopoietic recovery in UCBI+IST and IST groups
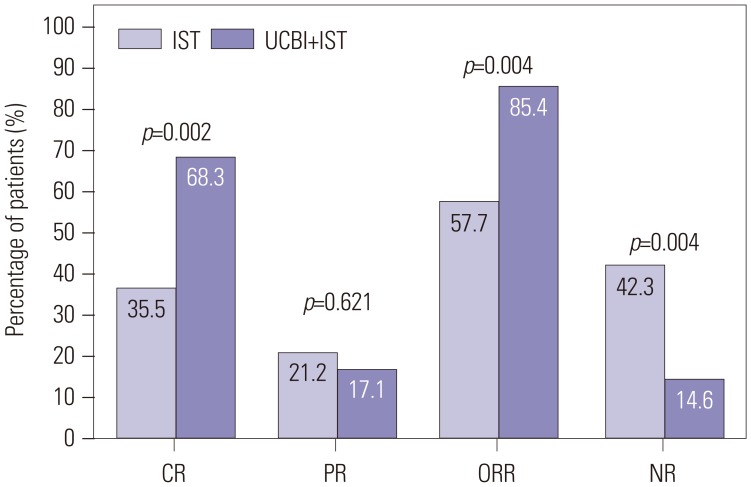
Fig. 2
K-M curves analysis for days to ANC, platelet, and hemoglobin responses in the UCBI+IST and IST groups. K-M curves revealed that patients in the UCBI+IST group achieved faster ANC (A) and platelet (B) responses, compared to the IST group. While no difference was discovered in hemoglobin response (C). Differences between groups were detected by K-M curves and log-rank test. p<0.05 was considered significant. K-M, Kaplan-Meier; ANC, absolute neutrophil count; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion.
OS analysis between UCBI+IST and IST treatment
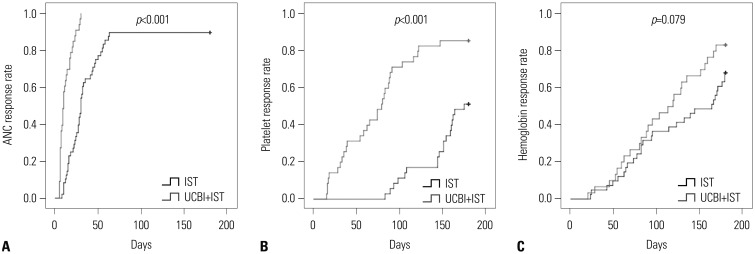
Fig. 3
Accumulating OS rates of patients in UCBI+IST and IST groups. No difference in accumulating OS rates between the two groups was found. Differences between groups were detected by Kaplan-Meier curve and log-rank test. p<0.05 was considered significant. OS, overall survival; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion.
Analysis of factors affecting CR
Table 3
Analysis of Factors Affecting CR
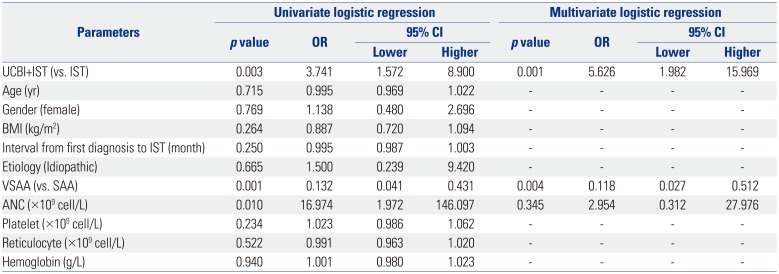
CR, complete response; CI, confidence interval; OR, odds ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate logistic regression was performed to analyze the factors affecting CR achievement, while factors with p value <0.1 was subsequently analyzed by multivariate model. p value <0.05 was considered significant.
Analysis of factors affecting ORR
Table 4
Analysis of Factors Affecting ORR
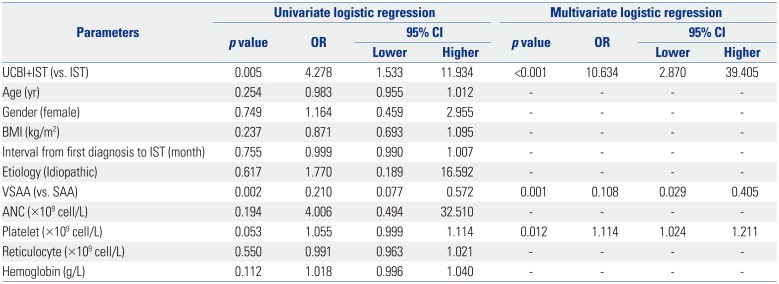
ORR, overall response rate; CI, confidence interval; OR, odds ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate logistic regression was performed to analyze the factors affecting ORR achievement, while factors with p value <0.1 was subsequently analyzed by multivariate model. in vivo value <0.05 was considered significant.
Analysis of factors affecting OS
Table 5
Analysis of Factors Affecting OS
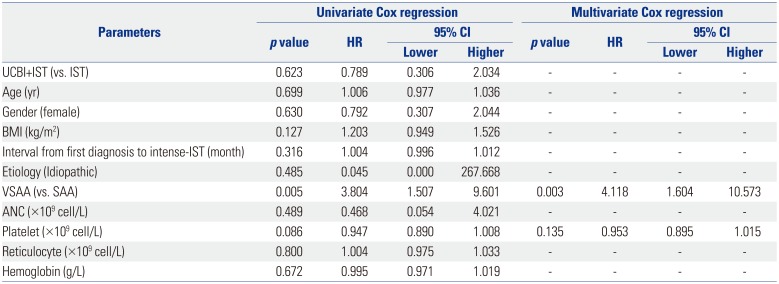
OS, overall survival; CI, confidence interval; HR, hazard ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate Cox proportional hazards regression was performed to analyze the factors affecting OS, while factors with p value <0.1 were subsequently analyzed by a multivariate model. p value <0.05 was considered significant.
Safety profiles of IST and UCBI+IST treatment
Fig. 4
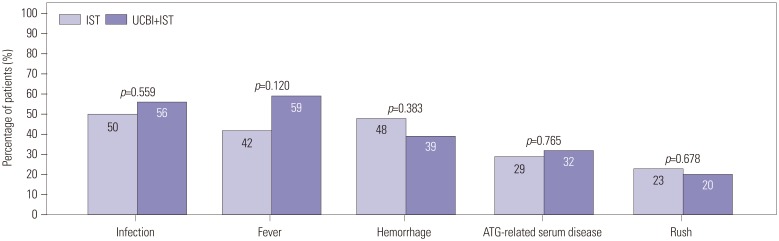
Comparison of adverse events between IST and UCBI+IST treatments. No differences were observed in infection, fever, hemorrhage, ATG-related serum diseases, and rush between IST and UCBI+IST groups. Differences between groups were evaluated by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ATG, antithymocyte globulin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download