Abstract
Purpose
Results on the clinical utility of cell therapy for ST-elevated myocardial infarction (STEMI) are controversial. This study sought to analyze the efficacy of treatment with intracoronary bone marrow mononuclear cells (BMMC) on left ventricular (LV) function and remodeling and LV diastolic and systolic function in patients with STEMI.
Materials and Methods
Literature search of PubMed and EMBASE databases between 2004 and 2017 was performed for randomized controlled trials in STEMI patients who underwent successful percutaneous coronary intervention and received intracoronary BMMC therapy. The defined end points were left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV). Also, sensitivity analysis and several subgroup analyses based on follow-up duration, timing of injection, doses of cells, and imaging modalities were conducted to strengthen the statistic power of the study.
Results
A total of 22 trials with 1360 patients were available for the current meta-analysis. The pooled statistics showed a significant improvement in LVEF {2.58 [95% confidence interval (CI), 1.32, 3.84]; p<0.001}, LVEDV [−3.73, (95% CI, −6.94, −0.52), p=0.02], and LVESV [−4.67, (95% CI, −7.07, −2.28), p<0.001] in the BMMC group, compared with the control group. However, in sensitivity analysis, a significant reduction in LVEDV disappeared, while the outcomes of LVEF and LVESV remained unchanged. The same results were presented in the subgroup analysis adjusting for imaging modalities and timing of cells injection.
Despite rapid advancements in both pharmacological and interventional treatment options, coronary heart disease remains the most common cause of death in Europe, accounting for 1.8 million deaths each year.1 Investigation of new therapies to improve left ventricular (LV) function and clinical outcomes after acute myocardial infarction (AMI) is actively ongoing. Experimental studies have been performed to investigate whether bone marrow cells (BMCs) transplantation post AMI could regenerate or repair damaged heart and vessels.23 The safety and feasibility of cell therapy has been established by a great many of studies using unselected bone marrow mononuclear cells (BMMC), progenitor cells, stem cells, or other mobilized cells.456 However, the clinical outcomes of each individual study have remained in controversy. Some studies have found positive effects for BMC therapy in the recovery of global and regional LV function. Others identified no favorable effects in a BMC group when compared with controls.
The conflicting results among trials may in part be explained by differences in patient selection, routes of cell administration, cell types, timing of cell infusion, dose of cells injection, and imaging modalities used in the evaluation of the treatment effect. To overcome the limitations of individual studies and increase statistical power, several meta-analyses have been performed.789 A meta-analysis in 2012 demonstrated that intracoronary BMCs therapy after AMI had a modest, but significant, improvement in left ventricular ejection fraction (LVEF) at 6 months after treatment (2.87%). Similar trends for LV end systolic volume (LVESV) and LV end diastolic volumes (LVEDV) were also concluded. Another meta-analysis in 2014 also showed modest increases in LVEF for the overall period (2.10%) and a reduction in LVESV. In contrast, a recent meta-analysis in 2015, selected individual patient data from 12 randomized clinical trials, and reported no beneficial effect at 1-year follow-up in LVEF, LVEDV, or LVESV. Taken together, these studies suggest that there remains a discrepancy in regards to the improvement of LV function.10 Furthermore, long-term follow-up data on LV diastolic function and LV systolic function are also important for further evaluation of safety and for more comprehensive understanding of the process of repairing and remodeling after AMI and BMCs therapy.
Accordingly, the current meta-analysis involved a large amount of studies focusing solely on patients with ST-elevated myocardial infarction (STEMI) treated with intracoronary infusion of BMMC after percutaneous coronary intervention (PCI) aiming to assess LV diastolic function and LV systolic function after cell therapy.
To identify relevant trials for our analysis, the electronic databases PudMed and EMBASE were searched with the following terms: bone marrow mononuclear cells, bone marrow cells, BMC, myocardial infarction, acute myocardial infarction, ST-elevation myocardial infarction, AMI, STEMI, cell therapy, randomized control trials, and all possible combinations. The included studies were limited to English and human experiments trials. Furthermore, reference lists of identified articles, recently published editorials, and reviews on the topic for further eligible trials were also hand searched by reviewers for additional studies. No duplicated data were used in this analysis.
The inclusion criteria were as follows: 1) randomized controlled trials (RCTs) comparing patients treated with BMMC and control therapy; 2) selected patients with STEMI; 3) patients under successful PCI before cell transplantation; 4) included proper outcomes of LVEF, LVEDV, and LVEDV; and 5) no other restrictions in terms of time, doses, or number of times of cell infusion.
The exclusion criteria were 1) nonhuman studies, 2) non-RCTs, 3) duplicated reports, 4) transplanted cells were not BMMC or circulating/ peripherals progenitor cells were mobilized by granulocyte colony stimulating factor from bone marrows, 5) lack of control group, and 6) no available data.
Data abstraction and analysis was performed by two different researchers independently and reported on standardized forms, including the first author, year of publication, patient population characteristics, study design (blinded or unblinded), injection time of cell therapy post STEMI, timing between cell aspiration and injection, type and dose of cells transplanted, measuring modality, follow-up duration, infracted territory, treatment option for control patients and cardiac parameters of LVEF, LVEDV, and LVESV. The mean changes in LVEF, LVEDV, and LVESV were taken as our primary endpoints. Data on cardiac parameters measured by echocardiography (ECHO), magnetic resonance imaging (MRI), LV angiography, and single photon emission computed tomography (SPECT) were considered equivalent. When two or more modalities were used and data were available, MRI data or ECHO data was preferentially used. Additional subgroup analyses were performed within the clinical trials investigating BMMC therapy in an attempt to gain more insight into possible discriminating parameters or differential conditions that might improve clinical outcomes in future experiments.
Subgroup analyses performed included 1) follow-up duration of 4 months, 6 months, 12 months, and 18 to 48 months; 2) different imaging modalities that were used to measure LVEF, LVEDV, and LVESV parameters; 3) BMMC injection time after STEMI onset symptom (within 24 hours, 2 to 14 days, and >14 days); and 4) doses of cells administrated (<10 millions, 10–100 millions, and >100 millions).
LVEF and LV volume were the primary end points of our analysis. In particular, we assessed the difference in mean changes (from baseline to follow up) of LVEF, LVEDV, and LVESV between patients receiving BMMC therapy and control treatment. We employed inverse-variance weighting to combine the results from independent studies. Most studies reported outcomes as mean difference (MD)±standard deviation (SD) at baseline and follow-up. The mean change of the outcome was then determined as MD at follow-up minus MD at baseline, whereas SD change was estimated according to the method described by Hristov, et al.11 Heterogeneity was analyzed with the I2 statistic and was defined as low (25–50%), intermediate (50–75%), or high (>75%). If p<0.05 or I2 >50%, a random effects model was used for data calculation. Otherwise, a fixed effects model was selected. When a considerable degree of heterogeneity was noted among trials (I2>50%), sensitivity analysis using the one study remove method and subgroup analysis was performed to seek out the source of heterogeneity. Pooled outcomes were displayed using forest plots, and we considered p values <0.05 (two-sided) as statistically significant. All statistical analyses were performed using RevMan 5.3 analysis software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
The search identified 934 potential publications, and we further screened these articles according to our inclusion and exclusion criteria. Based on the titles and abstracts of each text, 868 studies were excluded due to duplication and non-related topics. Sixty-six articles were applied for full-text analysis. Demographic characteristics and LVEF, LVEDV, and LVESV parameters were our outcomes of interest. Of the 66 studies that underwent full-text analysis, 44 studies were further excluded owing to other types of study subjects, regarding bone mesenchymal cells therapy (n=6), granulocyte-colony stimulating factor treatment (n=7), peripheral blood cells (n=4), lack of available data (n=15), and no baseline data or only treatment group data (n=12) (Fig. 1). Finally, 22 RCTs enrolling a total of 1360 participants, of which 704 patients were treated with BMMC therapy, were used in this meta-analysis.
The relevant study characteristics of each individual study that were thought to be an impact factor for the outcomes are summarized in Table 1. The recruited articles were published from 2004 to March 2015. Most studies used a 1:1 randomization scheme and studies sizes ranged from 10 to 176 patients. The follow-up duration ranged from 1 week to 48 months.12131415161718192021222324252627282930313233 Number of cells injected ranged from <108 to >109, and infusion time after STEMI onset was between less than 24 hours and 2 weeks later. Eighteen studies announced that patients suffered from STEMI of the anterior wall; another four did not clarify infarcted territory. Most of the studies performed BMCs aspiration and injection at the same day; two studies infused BMMC 24 hours after harvested. All of the selected trials applied freshly isolated BMMC by density gradient separation of autologous bone marrow aspirates, and cell injections were performed through intracoronary infusion after successful PCI post STEMI. All of the enrolled patients received BMMC infusion one-time-only. In the selected studies, LVEF, LVEDV, and LVESV were measured with MRI, SPECT, ECHO, and quantitative LV angiography. If one trial had applied several measurement tools, data measured by ECHO or MRI were preferentially used.
According to the combined data of 22 studies, cell therapy improved LVEF by 2.58 (95% CI, 1.32, 3.84; p<0.001; I2=71%) (Fig. 2), compared with controls. Due to the considerable high degree of heterogeneity among the studies, we conducted a sensitivity analysis using the one study remove method to seek out the heterogeneity sources, as well as to confirm the statistical power of the study. The initial result did not change when we omitted the studies one by one. However, we observed that, when removing the study by Piepoli, et al.25 and Schaefer, et al.,27 the I2 value changed from 71% to 60% and from 71% to 48%, respectively. When we omitted both studies, the I2 value further dropped from 71% to 25%. Therefore, we hypothesized the two studies might be the sources of heterogeneity.
The calculated data showed a significant reduction in LVEDV [−3.73, (95% CI, −6.94, −0.52), p=0.02, I2=58%] (Fig. 3). Because I2>50%, one factor at a time sensitivity analysis was also performed. When removing the studies by Cao, et al.15 and Huikuri, et al.,21 the only two studies that found significant improvement in LVEDV, the I2 value dropped to 0%. However, the positive effect of BMMC in LVEDV also disappeared [−1.24, (95% CI, −2.90, 0.43), p=0.14, I2=0%].
In the evaluation of LVESV, pooled data showed significant improvement in treatment groups, compared with controls [−4.67, (95% CI, −7.07, −2.28), p<0.001, I2=59%] (Fig. 4). The same sensitivity analysis was conducted, and no conflicting results were found. A decrease in I2 was observed went removing the studies by Piepoli, et al.25 and Schaefer, et al.,27 from 59% to 32%.
Pooled statistics revealed no beneficial effect toward BMMC groups at less than 6 months of follow up, when compared with control groups. The function of BMMC emerged at 6 months of follow up and sustained to 12 months by improving LVEF by 2.41 (95% CI, 0.39, 4.42; p=0.02; I2=88%) and 3.79 (95% CI, 2.72, 4.85; p<0.001; I2=18%), respectively. However, the favorable effect seemed to disappear at 18 to 48 months of follow-up [2.20 (95% CI, −0.28, 4.69; p=0.08; I2=79%)] (Fig. 5). Sensitivity analyses were performed in subgroups of 6 months and more than 12 months of follow-up. Again, no opposing outcomes were found when removing each study. On the other hand, when we deleted the results from Piepoli, et al.25 and Schaefer, et al.,27 the I2 value decreased in the 6-month group from 88% to 19%, and in the >12-month from 79% to 13%.
Subgroup analysis demonstrated that BMMC therapy was only effective at 3 months and 6 months of follow-up in the improvement of LVEDV, with −3.63 (95% CI, −6.57, −0.68; p=0.02; I2=0%) and −4.20 (95% CI, −8.11, −0.29; p=0.04; I2=70%), respectively (Fig. 6). The results of 12 months and 18–48 months follow-up did not show any significant difference between treatment group and controls. The study by Piepoli, et al.25 was found to cause high heterogeneity in sensitivity analysis.
The effect of cell therapy in LVESV manifested at 6 months follow-up −3.73 (95% CI, −6.23, −1.24; p=0.003; I2=56%) and at 12 months follow-up −7.93 (95% CI, −12.42, −3.44; p=0.0005; I2=75%) (Fig. 7). Whereas in short term follow-up of 3 months and 4 months, and in long term 18–48 months, BMMC treatment did not show superiority. As above, when removing the studies by Piepoli, et al.25 and Schaefer, et al.,27 the I2 value decreased to less than 50%.
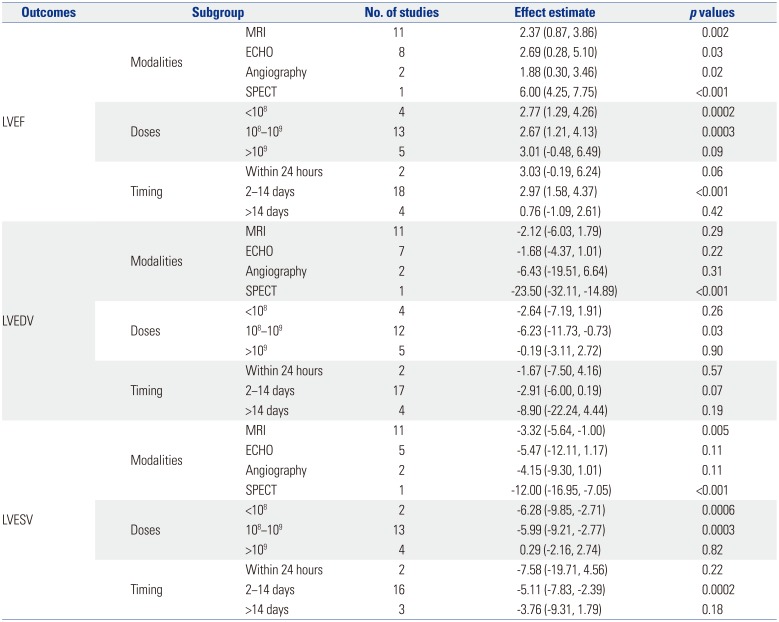
MRI is currently considered as the gold standard to assess LV function and volume. When subgroup analysis was performed based on MRI, the significant effect of BMMC therapy on LVEDV diminished [−2.12 (95% CI, −6.03, 1.79), p=0.29], while the significant improvement was still preserved in LVEF and LVESV (Table 2).
Based on our data, patients treated with an injection of more than 109 cells did not benefit more than patients with lower cell doses. More specifically, in studies that infused patients with less than 108 cells, LVEF and LVESV were significantly improved in treatment groups, compared with controls. Patients who received BMMC between 108 and 109 had significant improvement in LVEF, LVEDV, and LVESV. Whereas no such results were observed in the highest cell dose group (Table 2).
BMMC transplantation within 2 to 14 days after STEMI onset resulted in a significant elevation in LVEF by 2.97 (95% CI, 1.58, 4.37; p<0.001) and a decrease in LVESV by −5.11 (95% CI, −7.83, −2.39; p=0.0002). Cell therapy that was performed within 24 hours or more than 2 weeks after STEMI still improved LVEF, LVEDV, and LVESV. However, the outcomes did not reach statistical significant when compared with control patients (Table 2).
In this meta-analysis, we assessed LVEF, LVEDV, and LVESV as our primary end points to evaluate the effectiveness of BMMC therapy on LV function, LV remodeling, LV diastolic function and LV systolic function in patient post STEMI. The summarized data suggested BMMC therapy is associated with the recovery of LVEF and considerable reduction on LVEDV and LVESV. Due to the high degree of heterogeneity in each group, sensitivity analysis was conducted using the one study remove method. We assumed that two studies, Piepoli, et al.25 and Schaefer, et al.,27 might be sources of heterogeneity, as upon omitting the two studies in the analyses of LVEF and LVESV, the I2 value decreased to less than 50% while no original outcomes had changed. A comparison was made between the characteristics of the two studies and the others to disclose the reasons for the discrepancy. We found that the study by Piepoli, et al.25 was the only to use SPECT for outcome measurement. However, no major difference was found between Schaefer, et al.27 and other studies. Thus, the use of these two studies in future meta-analysis needs to be carefully deliberated. Interestingly, in LVEDV, when removing the studies by Cao, et al.15 and Huikuri, et al.,21 not only did the I2 value drop to 0%, the significant treatment effect on BMMC also disappeared. Therefore, the therapeutic effect of BMMC on LVEDV still remained controversial.
Whether cell therapy can provide a short-term or long-term effect is still in dispute. Many previous meta-analysis has explored this area. Cong, et al.34 reported that bone marrow stem cells was effective in the amelioration of LVEF, LVEDV, and LVESV at 3 to 12 months investigation. Chen, et al.35 suggested that cell transplantation only had beneficial effects on LVEF, which could last at least 2 years. De Jong, et al.8 concluded that intracoronary infusion of BMMC improved LVEF at 6 months to 12 months follow-up, mostly by reduction of LVESV. This conclusion is in accordance with our findings, which reflected a profound increase in LVEF and a reduction in LVESV at 6 to 12 months.
In the subgroup analysis adjusting imaging methods, when data were corrected for use of MRI, the positive effect of cell therapy on LVEDV diminished. More precisely, the treatment effect on LVEDV disappeared in all three imaging subgroups, MRI, ECHO, and angiography (Table 2). Already in sensitivity analysis when omitting the results of Cao, et al.15 and Huikuri, et al.,21 the effect on LVEDV became insignificant between BMMC and control patients. The finding of this subgroup further indicated that BMMC therapy might have no superior effect on LVEDV, compared with controls.
Prior studies put forward that poor cell engraftment and low survival of the transplanted BMMC are the major obstacles to the development of cell-based therapy.3637 They also suggested that only a small proportion of infusion cells remain in the heart,38 and most of them die after a few days.39 Therefore, many researchers hypothesized the number of injected cells might influence the treatment effect in STEMI patients. De Jong, et al.8 found that patients treated with an infusion of <100 million cells did not benefit more or less than patients with higher cell doses. On the contrary, the pooled outcome of our study suggested that patients who receive an infusion of 10 to 100 million cells might achieve the best therapeutic effect from BMMC.
The optimal time frame for intracoronary BMMC infusion was also assessed. Given the biological time course of healing and the expression of multiple factors, some researchers have believed that the highest probability for cells nesting and surviving was in the period between day 3 and day 7.40 Several meta-analyses already elucidated that BMMC transfer at 3 to 7 days post AMI was the optimal time to enhance cardiac function in patients.41 Our study provided further evidence that cell therapy performed within 2 to 14 days after symptom onset resulted in better recovery of LV function and volumes than patients receiving BMMC within 24 hours and after 14 days.
Some limitations need to be considered in this meta-analysis. Since the potential beneficial effects might be attributed to the combined effects of all infused mononuclear cells, rather than the small amount of progenitor cell or stem cell present in the bone marrow, our study restricted cell type to unselected BMMC. The effectiveness of other stem cell types, such as bone mesenchymal cells, remains to be established. Other factors, such as cells administrated routes, cell isolation protocols, patient characteristics, including age, gender, and weight, can have an influence on the effectiveness of cell therapy in STEMI patients. Future evaluations should put these factors into consideration. Our study only applied surrogate markers (LVEF, LVEDV, and LVESV) to estimate the therapeutic effect of BMMC. Despite the advantages of surrogate markers in the assessment of disease mechanisms, drug effect, and disease response, their use remains under scrutiny following the lack of some of the most reliable markers to predict clinical benefit of therapeutic interventions.42 A meta-analysis reported that BMC therapy was associated with long-term clinical benefits, such as all-cause mortality, although the effects on LV function and volumes were modest in short-term analyses.10 Future clinical trials should include clinical follow-up outcomes to validate the efficacy of cell therapy.
In conclusion, intracoronary infusion of BMMC leads to improvement of LVEF and reduction of LVESV, which indicates that cell therapy has a positive effect on LV function and can ameliorate adverse LV remolding. Further larger randomized multicenter trials with different cell types, injection methods, and timing and number of cell infusions are required to attain more accurate clinical evidence of cell therapy on STEMI.
References
1. Lang CI, Wolfien M, Langenbach A, Müller P, Wolkenhauer O, Yavari A, et al. Cardiac cell therapies for the treatment of acute myocardial infarction: a meta-analysis from mouse studies. Cell Physiol Biochem. 2017; 42:254–268. PMID: 28535507.

2. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. PMID: 9020076.

3. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410:701–705. PMID: 11287958.

4. Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, et al. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch Med Wochenschr. 2001; 126:932–938. PMID: 11523014.
5. Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003; 361:45–46. PMID: 12517467.

6. Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation. 2002; 106:3009–3017. PMID: 12473544.

7. Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015; (9):CD006536. PMID: 26419913.

8. de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014; 7:156–167. PMID: 24668227.
9. Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015; 116:1346–1360. PMID: 25700037.

10. Lee SH, Hong JH, Cho KH, Noh JW, Cho HJ. Discrepancy between short-term and long-term effects of bone marrow-derived cell therapy in acute myocardial infarction: a systematic review and meta-analysis. Stem Cell Res Ther. 2016; 7:153. PMID: 27765070.

11. Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med. 2006; 10:727–733. PMID: 16989732.

12. Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014; 35:1275–1283. PMID: 24569031.

13. Beitnes JO, Gjesdal O, Lunde K, Solheim S, Edvardsen T, Arnesen H, et al. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: a 3 year serial echocardiographic sub-study of the randomized-controlled ASTAMI study. Eur J Echocardiogr. 2011; 12:98–106. PMID: 20851818.

14. Benedek I, Bucur O, Benedek T. Intracoronary infusion of mononuclear bone marrow-derived stem cells is associated with a lower plaque burden after four years. J Atheroscler Thromb. 2014; 21:217–229. PMID: 24126180.

15. Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009; 30:1986–1994. PMID: 19508995.

16. Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown). 2011; 12:239–248. PMID: 21372740.

17. Dill T, Schächinger V, Rolf A, Möllmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009; 157:541–547. PMID: 19249426.

18. Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2011; 32:1736–1747. PMID: 21148540.

19. Hu X, Huang X, Yang Q, Wang L, Sun J, Zhan H, et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The CHINA-AMI randomized controlled trial. Int J Cardiol. 2015; 184:446–451. PMID: 25755063.

20. Huang R, Yao K, Sun A, Qian J, Ge L, Zhang Y, et al. Timing for intracoronary administration of bone marrow mononuclear cells after acute ST-elevation myocardial infarction: a pilot study. Stem Cell Res Ther. 2015; 6:112. PMID: 26021558.

21. Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008; 29:2723–2732. PMID: 18845667.

22. Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: doubleblind, randomised controlled trial. Lancet. 2006; 367:113–121. PMID: 16413875.

23. Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006; 355:1199–1209. PMID: 16990383.

24. Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006; 113:1287–1294. PMID: 16520413.
25. Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, et al. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (Cardiac Study). Eur J Heart Fail. 2010; 12:172–180. PMID: 20042424.

26. San Roman JA, Sánchez PL, Villa A, Sanz-Ruiz R, Fernandez-Santos ME, Gimeno F, et al. Comparison of different bone marrow-derived stem cell approaches in reperfused STEMI. A Multicenter, Prospective, Randomized, Open-Labeled TECAM Trial. J Am Coll Cardiol. 2015; 65:2372–2382. PMID: 26046730.
27. Schaefer A, Meyer GP, Fuchs M, Klein G, Kaplan M, Wollert KC, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from the BOOST trial. Eur Heart J. 2006; 27:929–935. PMID: 16510465.

28. Skalicka H, Horak J, Kobylka P, Palecek T, Linhart A, Aschermann M. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction and left ventricular dysfunction: a 24-month follow up study. Bratisl Lek Listy. 2012; 113:220–227. PMID: 22502753.
29. Srimahachota S, Boonyaratavej S, Rerkpattanapipat P, Wangsupachart S, Tumkosit M, Bunworasate U, et al. Intra-coronary bone marrow mononuclear cell transplantation in patients with ST-elevation myocardial infarction: a randomized controlled study. J Med Assoc Thai. 2011; 94:657–663. PMID: 21696072.
30. Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009; 30:1313–1321. PMID: 19208649.

31. Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011; 306:2110–2119. PMID: 22084195.
32. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364:141–148. PMID: 15246726.

33. Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009; 11:691–698. PMID: 19420003.

34. Cong XQ, Li Y, Zhao X, Dai YJ, Liu Y. Short-term effect of autologous bone marrow stem cells to treat acute myocardial infarction: a meta-analysis of randomized controlled clinical trials. J Cardiovasc Transl Res. 2015; 8:221–231. PMID: 25953677.

35. Chen L, Tong JY, Jin H, Ren XM, Jin H, Wang QJ, et al. Long-term effects of bone marrow-derived cells transplantation in patients with acute myocardial infarction: a meta-analysis. Chin Med J (Engl). 2013; 126:353–360. PMID: 23324289.
36. Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc. 2013; 2:e000338. PMID: 24113326.

37. Tongers J, Losordo DW, Landmesser U. Stem and progenitor cellbased therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011; 32:1197–1206. PMID: 21362705.

38. Penicka M, Widimsky P, Kobylka P, Kozak T, Lang O. Images in cardiovascular medicine. Early tissue distribution of bone marrow mononuclear cells after transcoronary transplantation in a patient with acute myocardial infarction. Circulation. 2005; 112:e63–e65. PMID: 16043651.

39. Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003; 1010:687–697. PMID: 15033813.

40. Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002; 55:329–340. PMID: 12123772.

41. Zhang S, Sun A, Xu D, Yao K, Huang Z, Jin H, et al. Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009; 32:458–466. PMID: 19685520.

42. Duivenvoorden R, de Groot E, Stroes ES, Kastelein JJ. Surrogate markers in clinical trials--challenges and opportunities. Atherosclerosis. 2009; 206:8–16. PMID: 19327774.

Fig. 1
Flow diagram of the literature search process and meta-analysis. BMMC, bone marrow mononuclear cell; G-CSF, granulocyte-colony stimulating factor; BMSC, bone mesenchymal cell.
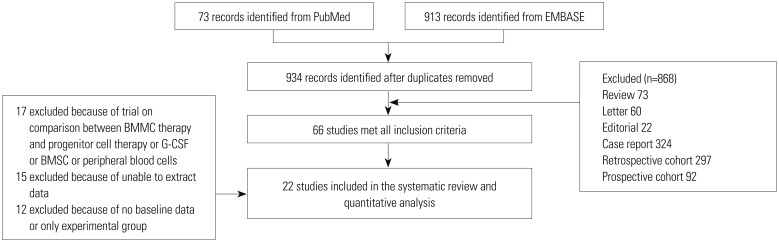
Fig. 2
Forest plot of the difference in change left ventricular ejection fraction from baseline to follow-up. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.
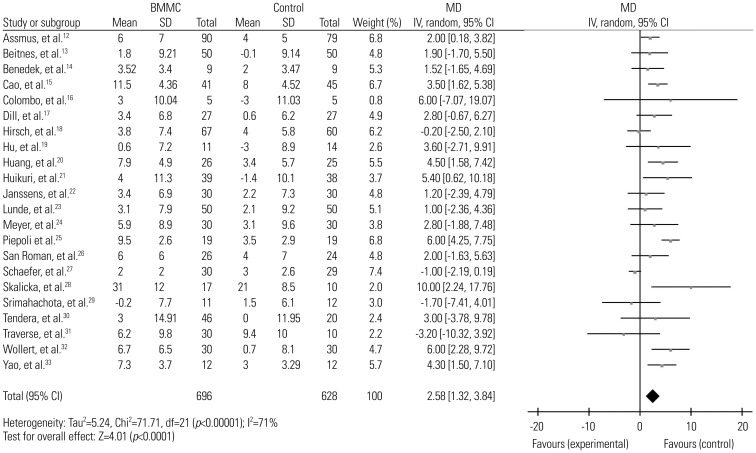
Fig. 3
Forest plot of the difference in change left ventricular end-diastolic volume from baseline to follow-up. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.
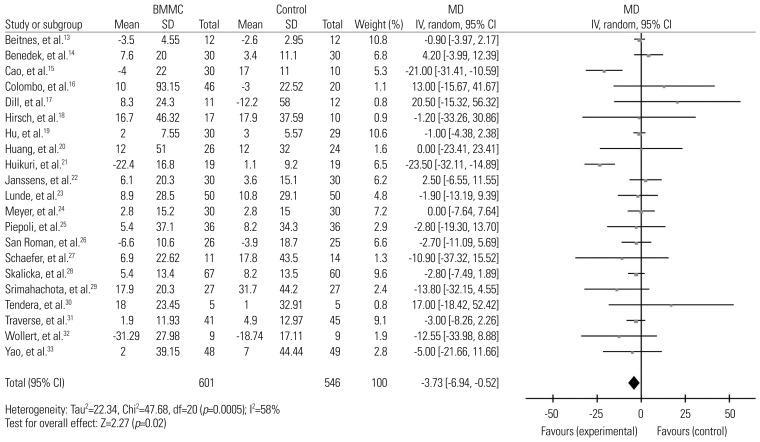
Fig. 4
Forest plot of the difference in change left ventricular end-systolic volume from baseline to follow-up. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.
Fig. 5
Forest plot of change in left ventricular ejection fraction of BMMC transplantation at different time durations. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.
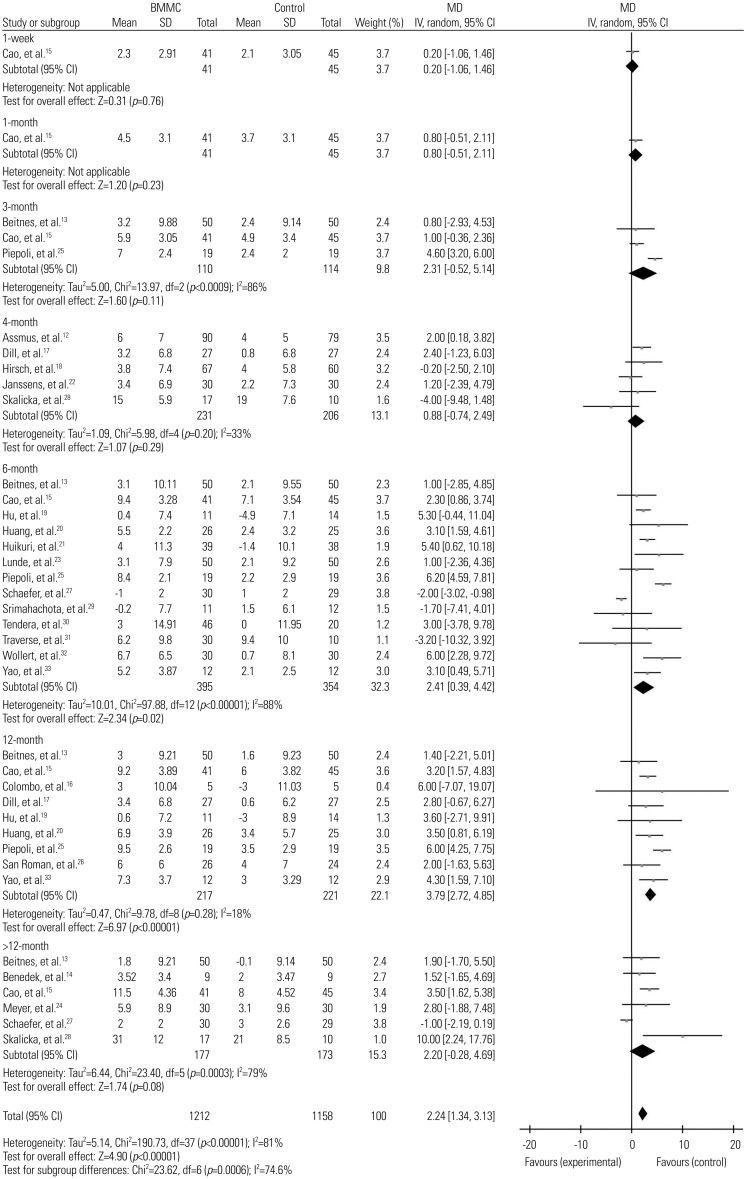
Fig. 6
Forest plot of change in left ventricular end-diastolic volume of BMMC transplantation at different time durations. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.

Fig. 7
Forest plot of change in left ventricular end-systolic volume of BMMC transplantation at different time durations. BMMC, bone marrow mononuclear cell; MD, mean difference; SD, standard deviation; CI, confidence interval.

Table 1
Population Characteristics
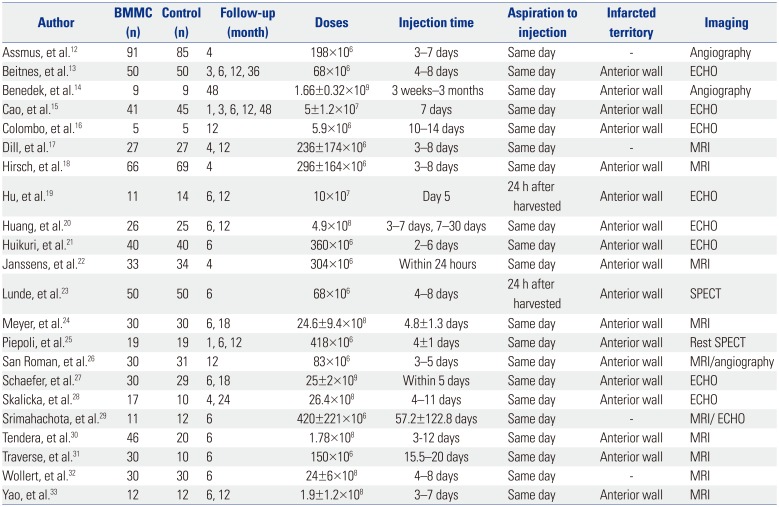
| Author | BMMC (n) | Control (n) | Follow-up (month) | Doses | Injection time | Aspiration to injection | Infarcted territory | Imaging |
|---|---|---|---|---|---|---|---|---|
| Assmus, et al.12 | 91 | 85 | 4 | 198×106 | 3–7 days | Same day | - | Angiography |
| Beitnes, et al.13 | 50 | 50 | 3, 6, 12, 36 | 68×106 | 4–8 days | Same day | Anterior wall | ECHO |
| Benedek, et al.14 | 9 | 9 | 48 | 1.66±0.32×109 | 3 weeks–3 months | Same day | Anterior wall | Angiography |
| Cao, et al.15 | 41 | 45 | 1, 3, 6, 12, 48 | 5±1.2×107 | 7 days | Same day | Anterior wall | ECHO |
| Colombo, et al.16 | 5 | 5 | 12 | 5.9×106 | 10–14 days | Same day | Anterior wall | ECHO |
| Dill, et al.17 | 27 | 27 | 4, 12 | 236±174×106 | 3–8 days | Same day | - | MRI |
| Hirsch, et al.18 | 66 | 69 | 4 | 296±164×106 | 3–8 days | Same day | Anterior wall | MRI |
| Hu, et al.19 | 11 | 14 | 6, 12 | 10×107 | Day 5 | 24 h after harvested | Anterior wall | ECHO |
| Huang, et al.20 | 26 | 25 | 6, 12 | 4.9×108 | 3–7 days, 7–30 days | Same day | Anterior wall | ECHO |
| Huikuri, et al.21 | 40 | 40 | 6 | 360×106 | 2–6 days | Same day | Anterior wall | ECHO |
| Janssens, et al.22 | 33 | 34 | 4 | 304×106 | Within 24 hours | Same day | Anterior wall | MRI |
| Lunde, et al.23 | 50 | 50 | 6 | 68×106 | 4–8 days | 24 h after harvested | Anterior wall | SPECT |
| Meyer, et al.24 | 30 | 30 | 6, 18 | 24.6±9.4×108 | 4.8±1.3 days | Same day | Anterior wall | MRI |
| Piepoli, et al.25 | 19 | 19 | 1, 6, 12 | 418×106 | 4±1 days | Same day | Anterior wall | Rest SPECT |
| San Roman, et al.26 | 30 | 31 | 12 | 83×106 | 3–5 days | Same day | Anterior wall | MRI/angiography |
| Schaefer, et al.27 | 30 | 29 | 6, 18 | 25±2×109 | Within 5 days | Same day | Anterior wall | ECHO |
| Skalicka, et al.28 | 17 | 10 | 4, 24 | 26.4×108 | 4–11 days | Same day | Anterior wall | ECHO |
| Srimahachota, et al.29 | 11 | 12 | 6 | 420±221×106 | 57.2±122.8 days | Same day | - | MRI/ ECHO |
| Tendera, et al.30 | 46 | 20 | 6 | 1.78×108 | 3-12 days | Same day | Anterior wall | MRI |
| Traverse, et al.31 | 30 | 10 | 6 | 150×106 | 15.5–20 days | Same day | Anterior wall | MRI |
| Wollert, et al.32 | 30 | 30 | 6 | 24±6×108 | 4–8 days | Same day | - | MRI |
| Yao, et al.33 | 12 | 12 | 6, 12 | 1.9±1.2×108 | 3–7 days | Same day | Anterior wall | MRI |
Table 2
Subgroup Analyses based on Imaging Modalities, Timing of Injection and Doses of Cells Infusion




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download