1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID:
28055103.

2. Resnick MI. Hormonal therapy in prostatic carcinoma. Urology. 1984; 24(5 Suppl):18–23.
3. Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942; 115:1192–1200. PMID:
17858048.

4. National Comprehensive Cancer Network. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN®): prostate cancer. ver. 2. Fort Washington (PA): National Comprehensive Cancer Network Inc;2017.
5. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002; 360:103–106. PMID:
12126818.

6. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006; 7:472–479. PMID:
16750497.

7. Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008; 300:173–181. PMID:
18612114.

8. Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008; 179:1830–1837. PMID:
18353378.

9. van den Bergh RC, van Casteren NJ, van den Broeck T, Fordyce ER, Gietzmann WK, Stewart F, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016; 69:802–820. PMID:
26691493.

10. Ryan CJ, Small EJ. Early versus delayed androgen deprivation for prostate cancer: new fuel for an old debate. J Clin Oncol. 2005; 23:8225–8231. PMID:
16278477.

11. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974. PMID:
9749478.
12. Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, Amin MB, et al. Prostate cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:245–253. PMID:
28222223.

13. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017; 71:630–642. PMID:
27591931.

14. Kirk D. Timing and choice of androgen ablation. Prostate Cancer Prostatic Dis. 2004; 7:217–222. PMID:
15278095.

15. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999; 341:1781–1788. PMID:
10588962.

16. Garcia-Albeniz X, Chan JM, Paciorek A, Logan RW, Kenfield SA, Cooperberg MR, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015; 51:817–824. PMID:
25794605.

17. Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016; 17:727–737. PMID:
27155740.

18. Fu AZ, Tsai HT, Haque R, Yood MU, Cassidy-Bushrow AE, Van Den Eeden SK, et al. Mortality and androgen deprivation therapy as salvage treatment for biochemical recurrence after primary therapy for clinically localized prostate cancer. J Urol. 2017; 197:1448–1454. PMID:
28007467.

19. Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004; 171:1141–1147. PMID:
14767288.

20. Taguchi S, Fukuhara H, Azuma T, Suzuki M, Fujimura T, Nakagawa T, et al. Ultra-early versus early salvage androgen deprivation therapy for post-prostatectomy biochemical recurrence in pT2-4N0M0 prostate cancer. BMC Urol. 2014; 14:81. PMID:
25323845.

21. Abugharib A, Jackson WC, Tumati V, Dess RT, Lee JY, Zhao SG, et al. Very early salvage radiotherapy improves distant metastasis-free survival. J Urol. 2017; 197(3 Pt 1):662–668. PMID:
27614333.

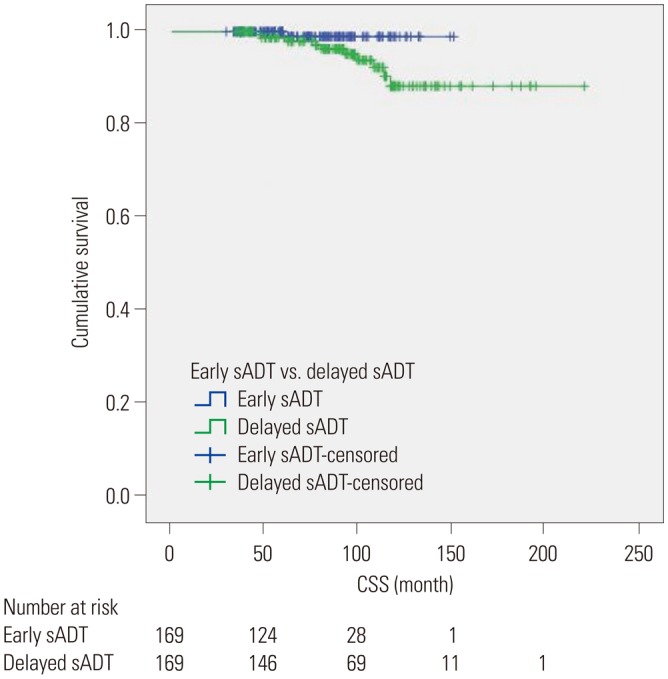
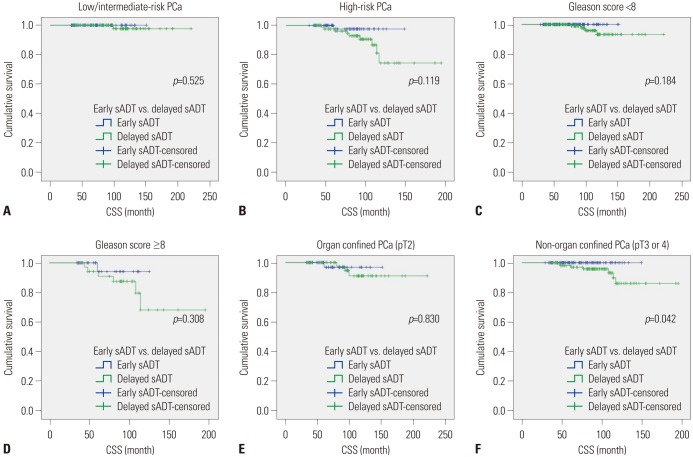
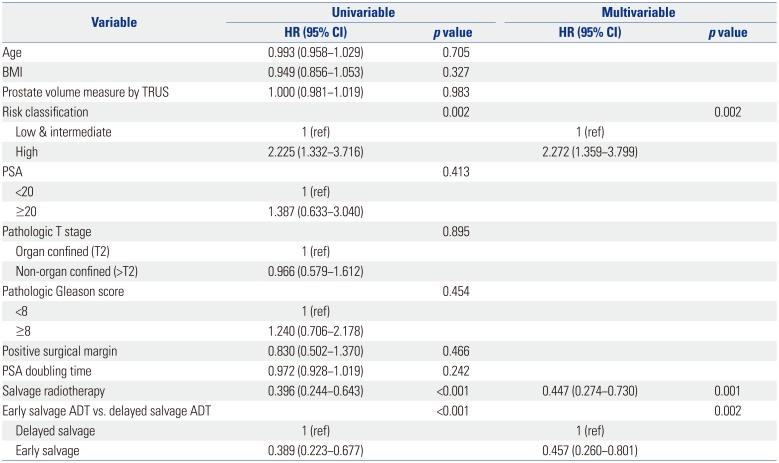
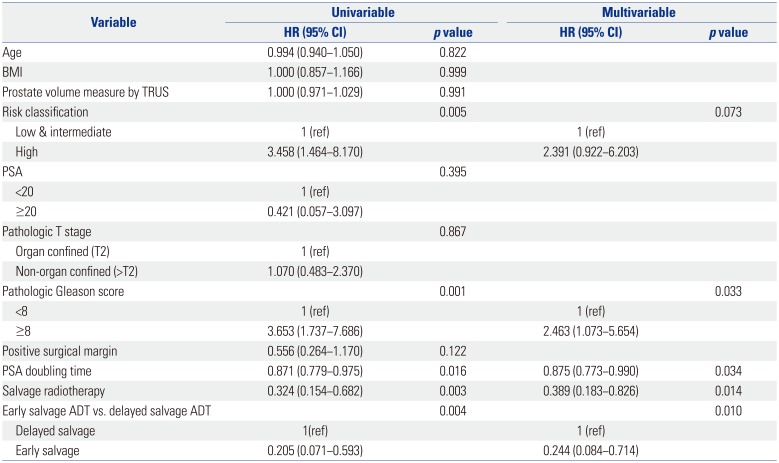




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download