Abstract
With advancements in diagnostic techniques, including molecular and clinical imaging, that directly target cancer cells, oligometastatic prostate cancer (PCa) is being diagnosed in patients who were, in the past, considered to have localized disease. With accumulating evidence, there has been a paradigm shift in considering aggressive treatments targeted at both the primary tumor and metastatic lesions in an aim to avoid and delay the need for palliative treatments and, ultimately, to achieve survival benefits. However, many questions still remain unanswered regarding the understanding of oligometastatic PCa, from its definition to optimal treatment strategies for each individual. Limited retrospective studies have suggested that interventions, including local and/or metastasis-directed therapy using surgery and radiation therapy (RT), can improve survival outcomes with minimal risk of adverse effects. Such treatments have been shown to decrease the risks of subsequent palliative interventions and to delay the start of androgen-deprivation therapy. Nevertheless, available data are insufficient to draw a reliable conclusion regarding their effect on quality of life measures and overall survival. This comprehensive review overviews data from contemporary literature that have investigated treatments, including surgery and RT, for patients with oligometastatic PCa, namely pelvic lymph node positive disease and limited distant metastases, and summarizes ongoing trials that are evaluating the feasibility of aggressive multimodal treatments.
Advances in clinical and molecular imaging techniques have led to the recognition of an intermediate state of prostate cancer (PCa) in which the disease has extended beyond the prostate, although with limited spread to distant organs. This state was first identified and given the terminology, oligometastasis, in 1995 by Hellman and Weichselbaum.1 Further research focusing on micro-RNAs involved in the development of metastatic disease has shown distinct regulation processes between oligometastatic and polymetastatic patients, suggesting that they are distinct disease entities rather than different points in a continuous evolution.2 Moreover, molecular pathological analysis with whole-genome sequencing tracing metastatic cell clones have suggested that the lethal clone may originate from the metastatic lesion, and not from the primary tumor.3 Thus, oligometastatic disease can be considered a heterogeneous disease entity with distinct metastatic phenotypes, which may have different prognoses. The clinical implication of this hypothesis is that definitive directed treatments for oligometastatic PCa, including radical prostatectomy (RP), radiation therapy (RT), and metastasis-directed surgery or ablative therapy, may be utilized for selected patients with a curative intent.
Emerging data suggest that treatment of the primary tumor and metastatic lesions with life-prolonging intent may improve survival for selected patients with metastatic PCa.4567 Traditionally, definitive treatments, such as RP or RT, were offered with curative intent only in the setting of a localized or locally advanced disease. Even minimal disease, such as a single positive pelvic lymph node (LN), precluded a definitive therapy, and these patients were treated with androgen-deprivation therapy (ADT).8 However, with emerging data that suggest treatments targeted at the primary tumor and the metastatic lesions may confer survival benefit, research is ongoing to distinguish subsets of patients who may benefit from aggressive treatment. Another advantage of metastasis-directed therapy would be a delay in the initiation of ADT. ADT remains a cornerstone of systemic therapy to alleviate cancer-related symptoms and to delay cancer progression.8 However, long-term use of ADT causes significant psychological and physical side effects, and has been suggested to decrease overall survival (OS) in some patients.9 In this aspect, the opportunity to perhaps delay or shorten the duration of ADT by treating oligometastatic PCa with curative intent seems clinically meaningful.
The curative treatment of oligometastatic PCa requires a three-tiered approach: 1) local consolidative therapy of the primary tumor, 2) metastasis-directed therapy, and 3) systemic chemohormonal therapy.10 To date, there is no definite evidence-based guideline on the role of these treatments for oligometastatic PCa. We performed a comprehensive review of contemporary literature to investigate current evidence regarding the feasibility of local treatment of the primary tumor and metastasis-directed therapy for patients with oligometastatic PCa.
The rationale of aggressive treatment for oligometastatic PCa stands on the hypothesis that such an approach may improve OS. Studies have observed that treatment of the primary tumor reduces the need for palliative interventions of locally advanced disease.1112 Furthermore, early local therapy has been found to delay the initiation of systemic therapies, such as ADT, which exerts detrimental effects on the quality of life.13
Several clinically and biologically plausible mechanisms have been proposed to explain how aggressive local therapy could improve survival in men with oligometastatic PCa. Paget, et al.14 introduced the concept of the “seed and soil” theory in 1889, a widely accepted mechanism in cancer biology. Thereafter, Kaplan, et al.15 described the perception of a “premetastatic niche” in which nonmalignant bone marrow-derived cells are able to sensitize the target tissue for circulating malignant cells to be recruited and develop metastatic deposits. In addition, the primary tumor may reciprocate with nonmalignant cells and endocrine factors to control the extent of metastasis. This was evident from bone marrow aspirates from patients with PCa, in which the development of metastasis was associated with the presence of circulating tumor cells in bone marrow only when the primary tumor was present. Moreover, despite the presence of circulating tumor cells, the risk of developing metastasis was imperceptible when the primary tumor was absent.16 Cytokine-based influence by the primary tumor was presumed for this observation. Recently, Abdollah, et al.17 observed that patients with LN positive PCa performed better with RT combined with ADT than ADT alone, supporting this hypothesis and providing the rationale of local treatment for oligometastatic PCa.
The feasibility of metastasis-directed therapy in patients with oligometastatic PCa is based on the notion that men with low-volume metastases will exhibit a more favorable outcome from less aggressive cancer biology, compared to patients with high-volume metastases. The concept of eradicating a small number of metastatic lesions stands on the hypothesis that it may yield improved systemic control and survival outcomes for oligometastatic PCa. Traditionally, tumor cell seeding had been considered a one-way process. However, Kim, et al.18 described a concept of “tumor self-seeding,” which suggests that circulating tumor cells can colonize their own origin of metastatic deposits. In other words, the primary tumor may act as a self-seeding site for circulating tumor cells to be primed and deposited at metastatic sites. This lends support to the notion that local therapy of the primary tumor or the metastatic deposits may inhibit the development of new metastases by altering the tumor microenvironment.
This theory has been clinically proven in metastatic settings of several cancers, including adrenal metastases from lung cancer, liver metastases from colorectal cancer, and lung metastases from various primary tumors.192021 The improvement in OS observed with these metastatic settings means that tumor debulking could derive similar benefits in PCa by prolonging the duration to a fatal tumor burden. Several related evidence also exists regarding metastatic PCa. Patients with low-volume metastases tended to progress locally instead of distant, while the opposite was observed for men with high-volume metastases.22 Moreover, the site and number of the metastases have been observed to affect survival outcomes in patients with metastatic PCa, suggesting that this concept may also be applied to PCa.23 Recently, investigators from the Mayo Clinic used stereotactic body radiation therapy (SBRT) for metastatic bone lesions of patients with oligometastatic castration-resistant PCa, and reported promising outcomes.24
Another potential advantage of metastasis-directed therapy is the delay of initiation of ADT, and the prevention of adverse effects associated with castrate levels of serum testosterone. The addition of metastasis-directed therapy to systemic treatment could also be an alternative active strategy that could eliminate the need or delay chemotherapy targeted at metastases and ultimately improve OS.25 The Munich registry also suggests that patients with metastasis who had RP had better survival, although a greater risk of postoperative incontinence was reported supposedly due to higher tumor burden often close to the sphincter.26 Indeed, such aggressive management could be considered for patients who refuse systemic therapy with a prioritization on quality of life measures.
For now, there is no consensus on whether treatment of the primary tumor with metastasis-directed therapy may confer improved survival based on an aggressive approach for oligometastatic PCa and can only be definitively obtained in the setting of randomized trials. This obstacle is augmented by the lack of a standard definition for oligometastatic PCa, which must be properly set out before we can examine the effects of therapy in the oligometastatic state.
The biological definition of oligometastatic PCa remains elusive, and improvements in imaging techniques are shifting the paradigm for conventional imaging. No formal cut-off for “oligo” has been defined, and should only be interpreted as a disease state between the presence of intravascular circulating tumor cells to disseminated metastasis.1023 Factors to consider when describing oligometastatic disease include the distinction of synchronous versus metachronous metastases, the number and site of lesions, the method of imaging, and whether the patient is castration-naïve or castration-resistant.27
Numerous studies have proposed different definitions regarding the number and sites of metastatic lesions based on oncological outcomes (Table 1), and as depicted, the optimal cut-off cannot be clearly determined.282930313233343536 Panels adjourned at the Advanced Prostate Cancer Consensus Conference 2017, but failed to reach a consensus on what constituted the definition of synchronous oligometastatic castration-naïve PCa.37 Specifically, 14% of the panels voted for two metastases, 66% for three metastases, and 20% voted for five metastases as a cut-off. Regarding the site of the metastases, 61% of the panels voted for a limited number of bone and/or LNs, 10% voted for a limited number of LN metastases, 13% voted for a limited number of metastases at any location including visceral disease, and 10% did not believe that oligometastatic PCa exists as a clinically meaningful entity.
In line with advancements in imaging techniques, more patients considered as M0 on conventional imaging will turn out to be oligometastatic. Further research is warranted for a clinically meaningful definition that influences treatment decisions and for the design of future clinical trials.
Since the 1990s, a survival benefit with RP has been suggested for patients with PCa involving only the pelvic LNs, compared to patients who received ADT alone.3839 Some observational studies have suggested an improvement in OS in patients with metastatic disease who had previously received RP.40 The mechanism underlying this survival advantage is unclear; however, according to the concept of oligometastatic disease described by Hellman and Weichselbaum,1 such approaches may have intervened the disease at a point of limited extent, and have controlled the metastatic “niche” to prevent further disease progression.
Contemporary literature on oncologic outcomes of patients with metastatic PCa has predominantly focused on men who received RP with LN dissection and were pathologically proven for LN metastasis. Reported 10-year cancer-specific survival (CSS) outcomes of these patients range between 70% and 85%.263941 However, these results were gained with the use of multidisciplinary strategies with adjuvant or salvage RT and ADT. Such promising results gained attention and encouraged the use of RP for selected LN positive patients. The Munich Cancer Registry was used to compare survival between patients with LN positive disease in whom RP was completed versus men in whom RP was aborted. Notwithstanding the limitation of a retrospective study, the multivariate model adjusted for confounders revealed RP to be independently associated with the risk of survival.27 These results were replicated by Steuber, et al.42 in which RP and positive LN burden were independently associated with CSS and clinical progression-free survival (PFS) in patients with LN metastasis who received adjuvant ADT. As such, it is important to note that not all patients with metastatic LNs may benefit from RP. A recent analysis of patients with LN positive PCa with a previous history of RP revealed higher cancer-specific mortality (CSM) in men with ≥3 positive LNs, positive surgical margins, Gleason score of 7 to 10, and absence of adjuvant RT.43 This highlights the importance of risk stratification in patients with oligometastatic PCa, which can aid in identifying those at risk of progression who may not be suitable candidates for aggressive multimodal treatment.
The benefit of RP can be cautiously inferred from two randomized trials that compared the effects of immediate versus delayed ADT with or without RP in patients with LN positive PCa.4445 The main difference between these trials was that the Eastern Cooperative Oncology Group (ECOG) 3886 trial included patients who received RP, while the ECOG 30846 trial included patients who received ADT alone. Both trials did not include patients with bone and visceral metastases. While the ECOG 30846 trial reported a 10-year OS rate of approximately 30% with ADT alone, the ECOG 3886 trial showed OS rates of 45% for RP alone and 64% for ADT in addition to RP.4445 Although these outcomes are from two studies with completely different cohorts, it provides evidence regarding the benefit of local treatment with surgery in men with limited LN metastasis.
Reports have shown that RP in patients with LN positive disease may offer improved oncologic outcomes when used as a multimodal strategy with adjuvant RT and ADT. Abdollah, et al.17 showed that adjuvant RT to RP with ADT in LN positive patients was significantly associated with a lower risk of CSM. However, at this time, there is insufficient evidence to set optimal timing and durations of neoadjuvant or adjuvant ADT.
Several studies have evaluated the role of RP in men with non-regional LN or bone metastatic PCa (Table 2). Sooriakumaran, et al.46 compared outcomes of patients with M1a/M1b disease who received RP and extended pelvic LN dissection with patients with M0 disease. The morbidity rates were comparable, with a CSS rate of 89% at 22.8 months in metastatic patients receiving RP. Jang, et al.47 retrospectively reviewed oncological and peri-operative outcomes between patients with five or fewer bone metastases without visceral metastases who received robot-assisted RP and those who received ADT alone. Robot-assisted RP was a significant predictor of both PFS (hazards ratio=0.39) and CSS (hazards ratio=0.26), with comparable postoperative complications with previously reported RP-treated patients.
The first case-controlled study was performed for patients with low-volume M1b PCa, defined as ≤3 bone metastases on bone scan. Patients who received cytoreductive prostatectomy with extended pelvic LN dissection were compared to a control group treated with ADT alone. Compared to non-RP group, the RP group demonstrated significantly longer clinical PFS (39 vs. 28 months), increased time to castration-resistance (40 vs. 29 months), and improved CSS (96% vs. 84%). However, the median CSS period was comparable (47 vs. 41 months). Of note, all surgical patients were responsive to ADT, while 32% of the control patients did not respond to ADT, suggesting a potential selection bias.12
The Surveillance, Epidemiology, and End Results (SEER) data were utilized to compare survival outcomes in patients who underwent RP or brachytherapy to patients who did not receive local therapy.48 Baseline differences in clinicopathological features existed between the two groups, with untreated patients being older and comprising a greater proportion of Gleason scores >8 PCa and a smaller proportion of cN0 disease. Nevertheless, multivariate competing risk regression analysis revealed that patients treated with RP exhibited a 62% decreased risk of CSM, compared to controls. Of note, the benefit of RP in regards to improved CSS was observed across all M stages.48 The SEER data was revisited by Antwi and Everson49 using a propensity-score matched analysis to ensure adjustments in baseline cohort differences. In accordance to the previous study, improved CSS was observed for RP for all M stages. Another population-based study was conducted using the Munich Cancer Registry to compare treatment outcomes of patients with metastatic PCa treated with RP versus non-RP patients treated with primary ADT or RT. Patients who received RP exhibited a significantly higher 5-year OS, compared to their counterparts (55% vs. 21%).50 Considering their retrospective, population-based nature, these results should be cautiously interpreted. However, the consistent findings from sophisticated analyses lend support to the notion that RP may be a feasible option for men with de novo distant metastatic PCa.
Considering the protracted natural history of PCa, Satkunasivam, et al.51 linked the SEER database to the Medicare database to account for comorbidities and the use of ADT, which may affect survival. The study cohort included patients who received RP, intensity modulated RT, conformal RT, and no treatment. The RP group was younger, had lower serum prostate-specific antigen (PSA) levels, T and N stages, and Gleason score PCa than those who did not receive local treatments, and were less likely to receive bone radiation or ADT in the 6-month period following diagnosis. On multivariable analysis adjusted for ADT, tumor characteristics, comorbidities, and RT to bone within 6 months of diagnosis, RP conferred a 52% decrease in the risk of CSM.
Two clinical trials are currently evaluating the benefit of surgery in patients with oligometastatic PCa. The first is a nonrandomized trial comparing CSS and OS outcomes between patients receiving RP with extended LN dissection and those receiving non-surgical interventions as local treatment (NCT02138721).52 The second will determine the safety and feasibility of cytoreductive prostatectomy with adjuvant ADT in patients with newly diagnosed metastatic PCa (NCT02458716).53
Salvage LN dissection, if all disease can be removed, can be considered for patients with recurrent LN disease following primary local therapy. The decision is a challenging process and the correct localization of recurred site is essential in the clinical decision making. Advanced imaging modalities, such as 11C-choline or prostate-specific membrane antigen (PSMA) positron emission tomography with coregistered computed tomography (PET/CT) may allow selection of optimal candidates for salvage LN dissection.
Several limited case series have evaluated the benefit of salvage LN dissection.545556 Karnes, et al.54 observed that 57.7% of patients showed PSA levels <0.2 ng/mL and that 75% of patients remained free of systemic progression during the median follow-up of 20 months. Of note, 83% of the patients had received adjuvant ADT following surgery. Suardi, et al.56 observed PSA levels <0.2 ng/mL in 59% of men at 40 days, an 8-year biochemical recurrence (BCR)-free survival rate of 23%, and a CSS rate of 81%. Of note, ADT was given if the patient had BCR. At a 5-year follow-up, 40% of men were free from ADT. Jilg, et al.55 recorded that 46% of patients exhibited a complete biochemical response, with an 1-year BCR-free survival of 71.8% and a 5-year CSS rate of 77.7%. Multivariate analyses from these studies indicated that patients with PSA level <4 ng/mL at LN dissection, Gleason score <8, and no involvement of retroperitoneal LNs are better candidates for salvage LN dissection.
A systematic review of 12 observational studies regarding salvage LN dissection with immediate or deferred ADT has reported a 5-year OS rate of approximately 75% and BCR-free survivals ranging between 9% and 22%. Favorable prognosticators following salvage LN dissection were patients with complete PSA response, fewer positive LNs, no involvement of retroperitoneal LNs, and lower preoperative PSA.57 It is important to note that these data were derived from observational studies, and there were no control groups who received ADT alone or did not receive salvage LN dissection. Future studies are warranted to determine whether these survival outcomes are the result of salvage LN dissection or the reflection of the natural history of oligometastatic recurrent nodal disease.
Radiation therapy plays an important role within the treatment landscape of PCa, ranging from up-front treatment for localized disease with curative-intent, post-RP adjuvant or salvage settings, and as a palliative treatment for men with symptomatic metastases.25 Clinical studies on RT used for oligometastatic PCa in improving OS or other clinical endpoints have focused on both conventional external beam RT (EBRT) and SBRT. Studies have suggested that the mechanism of action of SBRT is distinct from EBRT. SBRT can precisely target the lesion with an intensified radiation dose while minimizing the radiation exposure to adjacent normal tissues.58 Moreover, the low α/β value of PCa enables hypofractioned SBRT to effectively eradicate the target lesions.59 Compared to EBRT, which delivers localized cytotoxic effects, SBRT has been shown to induce immune responses with the potential for additional tumor control.6061
Three and seven studies have described the roles of EBRT and SBRT as metastasis-directed therapies after local treatment, respectively (Table 3). Tabata, et al.36 investigated survival outcomes of men with ≤5 oligometastases or oligo-recurrence of bone metastatic PCa treated by EBRT at a median dose of 40 Gy. The 3-year OS rates for all patients, for patients that received a dose of ≥40 Gy, and for those that received <40 Gy were 77%, 91%, and 50%, respectively. Of note, 87.5% of patients demonstrated relief of pain at 1 month, and pathological fracture and spinal cord compression did not occur at the irradiated sites. Rades, et al.62 investigated outcomes of 133 patients with metastatic spinal cord compression with ≤3 vertebral lesions detected by CT or MRI and treated with EBRT alone. For patients with PCa, the 2-year actuarial local control, defined as recurrence of motor deficits in the previously irradiated spinal region, and OS rates were 80% and 62%, respectively. Schick, et al.34 investigated the feasibility of EBRT to treat patients with ≤five distant and/or regional LNs, bone, and/or lung lesions detected by 18F-choline 11C-acetate PET-CT. During the median followup of 31 months, the authors observed a 3 year BCR-free survival of 55%, clinical failure-free survival of 59%, and an OS of 92%. Of note, neoadjuvant and concomitant ADT was given to all but one patient.
Studies addressing RT as a metastasis-directed therapy have mainly focused on SBRT. The first studies for SBRT were reported in 2009 by Jereczek-Fossa, et al.,63 who described outcomes of men with isolated LN metastasis detected by choline PET/CT and treated with CyberKnife image-guided SBRT. At a mean follow-up of 18.6 months, the local control rate was 100%. Later, the same methodology was revisited with a larger cohort of patients with local recurrence, single LN metastasis, and single distant metastasis. The 30-month PFS rate was 42.6%.64 Similarly, Muacevic, et al.65 prospectively enrolled a total of 40 patients with one or two bone metastases and evaluated the feasibility of a single fractional CyberKnife robotic radiosurgery. Local control, defined as no tumor growth on MRI and lack of increased tracer uptake on choline PET-CT, was achieved in 95.5% of patients at 2-year follow-up. Of note, 68% of men were treated with ADT during follow-up. Berkovic, et al.29 reported outcomes of patients who received SBRT at a dose of 50 Gy in 10 fractions for ≤3 bone or LN metastases detected with 18F-PET or 11C-acetate PET. The 2-year local control and clinical progression-free survival was 100% and 42%, respectively. 42% of patients started ADT due to progression of metastasis and PSA elevation, resulting in a median ADT-free survival of 38 months. Notably, repeated salvage SBRT for metachronous disease was feasible, without significant toxicity. A similar study was performed using SBRT to target liver and LN lesions in addition to bone. Local control, defined as lack of tumor progression, was achieved in all patients at a median followup of 6 months. Among these patients, 53% of men reached undetectable serum PSA levels. Of note, 55% of men with CRPC exhibited undetectable PSA levels or had persistently declining PSA at the time of analysis. No Grade 3 or late toxicities were observed.27 Decaestecker, et al.30 described outcomes for 50 men with ≤3 synchronous LN, bone, or liver metastases treated with repeated SBRT at a dose of 50 Gy in 10 fractions or 30 Gy in three fractions. The PFS rate at 2 years was 35%, while the local control rate was 100 months. The median delay to ADT was 25 months. Of note, PSA doubling time was a prognosticator of clinical progression and ADT-free survival. Lastly, a multi-institutional retrospective review evaluated distant PFS of patients with ≤3 metachronous metastatic lesions in which SBRT was delivered to all lesions. Distant metastatic lesions were detected using 18F-PETor choline PET. Herein, a median of 21-month distant PFS was reported, with concurrent ADT not having a significant benefit in survival.32
In summary, SBRT demonstrates excellent local control, with delay in both biochemical and clinical progression. The tolerability of SBRT seems to be acceptable, without significant grade III toxicity. The use of SBRT to delay ADT confers the benefit of maintaining a good quality of life. However, it is more likely that SBRT will exert synergistic effects when combined with systemic therapy, which may result in improved oncological outcome. In order to define the potential benefits of SBRT on long-term oncological outcomes and to establish a standard of care, larger studies with homogeneous cohorts will be required.
Traditionally, positive pelvic LNs or distant metastasis preclude a definitive therapy and are treated with ADT. In an aim to improve survival outcomes for this disease setting, attempts to combine ADT with RT have been made and reported since early 2000 (Table 4).
To date, no randomized controlled trial has compared ADT combined with RT to the primary tumor versus ADT alone in metastatic PCa. Zagars, et al.66 reported a retrospective analysis on oncologic outcomes of positive LN patients treated by RT combined with ADT or ADT alone. The pelvic LNs were not included in the RT field. During the median follow-up of 6.2 years, the RT combined with ADT group exhibited better biochemical-free, metastasis-free, and OS outcomes, compared to the ADT alone group. Two randomized controlled trials have compared ADT and RT of the prostate versus ADT alone in a large group of men with locally advanced or high-risk localized PCa without distant metastasis. The addition of local RT to ADT was associated with reductions in overall mortality and CSM. The addition of RT also showed a benefit in regards to disease PFS. The adverse effects of RT were acceptable, and the frequency of serious toxicity was low.6768 A phase III, randomized trial was performed to compare OS and metastasis-free survival of patients with radiographic or pathologic LN metastasis who received RT with immediate ADT or RT alone and ADT at relapse. During the median follow-up of 6.5 years, RT with immediate ADT conferred superior survival, compared with RT alone and ADT at relapse, suggesting the importance of immediate ADT in LN positive patients.69 Lastly, the National Cancer Database was used to investigate OS in patients with de novo metastatic PCa receiving ADT with and without RT on the prostate. On propensity score-matched analysis adjusted for baseline clinicopathological features, the ADT plus RT group demonstrated superior 5-year OS, compared to the ADT alone group (49% vs. 33%).70 These results provide evidence that aggressive local control of the primary tumor may help to eradicate future metastatic clones and ultimately improve survival.
Until recently, ADT was regarded as the standard of care for metastatic castration-sensitive PCa. However, evidence from recent trials has changed this paradigm and supports the advantage of systemic chemohormonal therapy in the oligometastatic setting.
STAMPEDE was a randomized, multi-arm, and multi-stage trial that recruited patients who were started on first-line ADT for de novo metastatic, LN positive, or high-risk locally advanced disease or previously treated with RP or RT and relapsing with high-risk features. The OS benefit of adding zoledronic acid and/or docetaxel to ADT was investigated. Zoledronic acid demonstrated no evidence of OS benefits, while docetaxel chemotherapy conferred improved OS. Based on the results from this trial, docetaxel chemotherapy was suggested to be a standard of care for chemo-fit men with metastatic disease and for men with high-risk non-metastatic PCa with or without RT.71
A systematic review and meta-analysis were performed on three eligible trials, including PEACE-1 (NCT01957436),72 LATITUDE (NCT01715285),73 and STAMPEDE (NCT00268476),74 all of which assessed the benefit of adding abiraterone acetate plus prednisone/prednisolone (AAP) to ADT in patients with metastatic castration-sensitive PCa. The results showed a 38% reduction in the risk of death with AAP plus ADT, which translated into a 14% gain in 3-year OS. Moreover, a 55% reduction in the risk of PFS was observed with the addition of AAP, which translated to a 28% improvement at 3 years. The results provided evidence to suggest that AAP with ADT may be an alternative option to upfront docetaxel, with potential survival benefits for patients with metastatic castration-sensitive PCa.75
The STAMPEDE and PEACE-1 have evolved the paradigm for systemic chemohormonal therapy for oligometastatic PCa, and ongoing clinical trials are investigating the synergistic effect of ADT combined with agents, including abiraterone acetate, enzalutamide, palbociclib isethionate, orteronel, apalutamide, and docetaxel.25
Defining the optimal subgroup of patients with oligometastasis for aggressive multimodal therapy is difficult. Generally, patients with longer life expectancy, good performance status, and few comorbidities would be suitable candidates.7677 The lesion that will be targeted by either surgery or radiotherapy should be in a suitable location with a reasonable size so that the treatment will not cause detrimental consequences.
Current clinical practice is to define the extent of PCa with 99mTechnetium-methylene diphosphonate (99mTc-MDP) bone scan, as well as computed tomography (CT) or magnetic resonance imaging (MRI) to determine the extent of disease in men with PCa. These conventional imaging modalities, however, are limited in terms of their sensitivity for detecting small volume sites of PCa and may underestimate the burden of disease.78 More specifically, bone scans are estimated to have a sensitivity of only 65%, suggesting that a significant amount of metastatic disease may go undetected.79 In addition, it is known that the majority of LN metastases will not meet established size criteria for detection with CT or MRI in those patients undergoing preoperative staging.80 Due to these limitations, more sensitive imaging tests are needed to accurately establish extent of metastatic spread in patients with PCa.
During the past few years, advances in molecular and clinical imaging that directly target cancer cells have conferred a more efficient and potentially sensitive approach for PCa imaging. The higher yield of detecting lymphatic and/or hematogenous metastases at an earlier stage of the disease progression has resulted with a treatment window for oligometastatic disease, and at the same time, is continuously refining the definition of oligometastatic PCa.10
Imaging modalities that have recently received attention include 11C-choline PET/CT, PET/MRI, 18fluorodihydrotestosterone PET, 68Ga-labelled PSMA, combined ultra-small superparamagnetic particles of iron oxide-enhanced, and diffusion-weighted (USPIO-enhanced MRI) and ferumoxytol enhanced MRI.102381 Among these, PSMA-targeted imaging has recently gained particular interest, as radiotracers targeting this cell surface protein have been shown in numerous reports to offer outstanding sensitivity for detecting small-volume sites of PCa at low PSA levels that are not detectable on conventional imaging.82 Furthermore, the addition of MRI has the potential to integrate the diagnostic path in cases with moderate PSMA tracer accumulation.83 Emerging imaging modalities for staging PCa seems promising and will have an increasing role in defining oligometastatic PCa and selecting the optimal candidates for aggressive treatments.
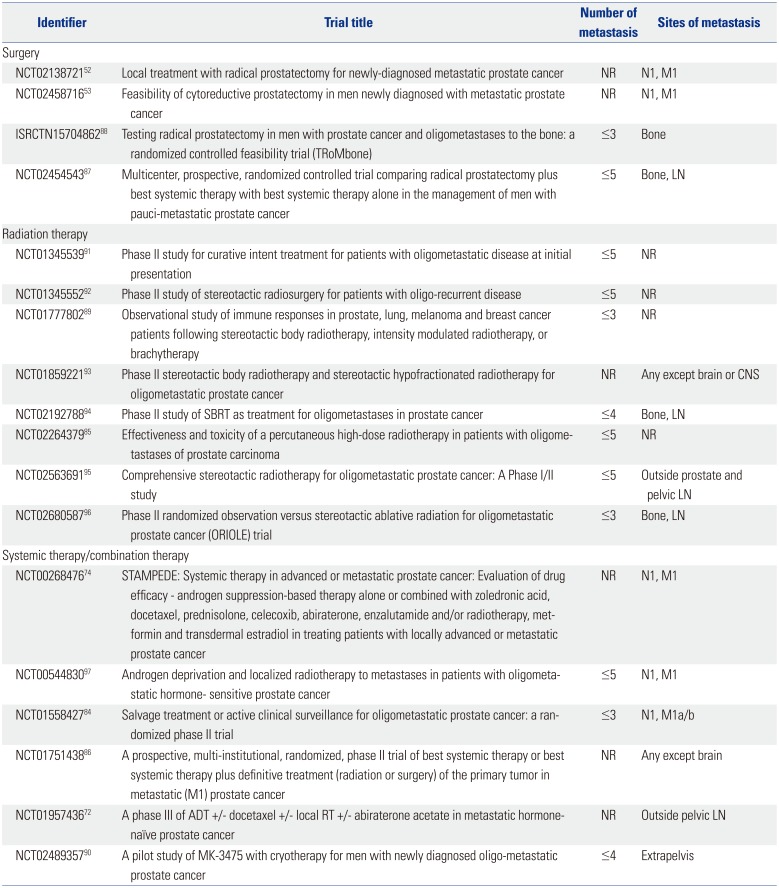
High level evidence needs to be obtained through clinical trials to establish a standard of care. At this point, several active clinical trials are investigating the role of multidisciplinary treatments for oligometastatic, de novo metastatic, or oligorecurrent PCa (Table 5). Most of the trials are evaluating the role of radiation therapy, specifically stereotactic radiotherapy. A randomized, phase II trial is evaluating the efficacy of metastasis-directed therapy with combined surgery or SBRT in oligometastatic recurrence after local therapy versus active surveillance (NCT01558427).84 One trial compares toxicity rates between normal fractionated versus hypofractionated radiation (NCT02264379).85
Trials are evaluating the efficacy of local therapy in patients with newly diagnosed metastatic or oligorecurrent PCa (NCT00268476, NCT01751438, NCT01957436, NCT02138721, NCT02454543, and ISRCTN15704862).527274868788 The TRoMbone trial randomizes 50 men to standard-of-care using ADT with or without docetaxel versus standard-of-care plus RP with extended pelvic LN dissection (ISRCTN15704862).88
Targeting the anticancer immune response has shown promise in melanoma, bladder, and lung cancers, suggesting that immunotherapies could also be effective in oligometastatic PCa. An observational trial is recruiting patients with or without primary treatment managed with SBRT to observe the induction of anti-PCa immunity (NCT01777802).89 Another trial is recruiting patients for combined primary treatment of the tumor with immunotherapy (NCT02489357).90 There is lack of data regarding oligometastatic PCa whether definitive treatment of the primary tumor may improve OS, such as for cytoreductive nephrectomy in patients with metastatic renal cell carcinoma. One trial is evaluating the efficacy of cytoreductive surgery in de novo metastatic PCa (NCT02138721).52
Since defining the oligometastatic state of PCa, a paradigm shift towards more aggressive treatment is gaining support. Emerging data suggests treatments targeted at the primary tumor and the metastatic lesions in patients with oligometastatic PCa may prevent or delay the need for palliative treatments and confer survival benefit. Among several treatment modalities, SBRT has been demonstrated to be safe and well tolerated, with excellent local control of bone and LN metastases. The biology underlying metastatic progression from the primary tumor to the metastatic sites, or vice versa, is not completely understood, and future research is needed to comprehend the exact nature of these potentially distinct disease entities. Molecular imaging represents a major development and an essential tool in the staging of patients with oligometastatic PCa. In particular, 68Ga PSMA is a promising tracer that can accurately assess disease burden, and can be utilized to select optimal candidates and tailor treatments. At this time, there is no consensus on performing metastasis-directed therapy for oligometastatic PCa in order to delay ADT and improve survival. However, it can be considered in highly selected patients who demonstrate an indolent course with a limited disease burden. Systemic therapy with ADT should be recommended for patients with a higher disease burden, with the addition of docetaxel for chemotherapy-fit patients. Results from ongoing prospective clinical trials will be needed to better define oligometastatic PCa and to clarify the rationale of aggressive treatment.
References
2. Uppal A, Ferguson MK, Posner MC, Hellman S, Khodarev NN, Weichselbaum RR. Towards a molecular basis of oligometastatic disease: potential role of micro-RNAs. Clin Exp Metastasis. 2014; 31:735–748. PMID: 24968866.

3. Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013; 123:4918–4922. PMID: 24135135.

4. James ND, Spears MR, Clarke NW, Dearnaley DP, Mason MD, Parker CC, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE Trial. JAMA Oncol. 2016; 2:348–357. PMID: 26606329.
5. Tward JD, Kokeny KE, Shrieve DC. Radiation therapy for clinically node-positive prostate adenocarcinoma is correlated with improved overall and prostate cancer-specific survival. Pract Radiat Oncol. 2013; 3:234–240. PMID: 24674370.

6. Rusthoven CG, Carlson JA, Waxweiler TV, Raben D, Dewitt PE, Crawford ED, et al. The impact of definitive local therapy for lymph node-positive prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014; 88:1064–1073. PMID: 24661660.

7. Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015; 67:852–863. PMID: 25240974.

8. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005; 294:238–244. PMID: 16014598.

9. Kim J, Park JS, Ham WS. The role of metastasis-directed therapy and local therapy of the primary tumor in the management of oligometastatic prostate cancer. Investig Clin Urol. 2017; 58:307–316.

10. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017; 14:15–25. PMID: 27725639.

11. Won AC, Gurney H, Marx G, De Souza P, Patel MI. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013; 112:E250–E255. PMID: 23879909.

12. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015; 193:832–838. PMID: 25254935.

13. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009; 115:2388–2399. PMID: 19399748.

14. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989; 8:98–101. PMID: 2673568.
15. Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006; 66:11089–11093. PMID: 17145848.
16. Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009; 27:1549–1556. PMID: 19237635.

17. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Predicting survival of patients with node-positive prostate cancer following multimodal treatment. Eur Urol. 2014; 65:554–562. PMID: 24094576.

18. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009; 139:1315–1326. PMID: 20064377.

19. Strong VE, D'Angelica M, Tang L, Prete F, Gönen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007; 14:3392–3400. PMID: 17665267.

20. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318. PMID: 10493478.
21. Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997; 113:37–49. PMID: 9011700.

22. Furuya Y, Akakura K, Akimoto S, Inomiya H, Ito H. Pattern of progression and survival in hormonally treated metastatic prostate cancer. Int J Urol. 1999; 6:240–244. PMID: 10375186.

23. Aoun F, Peltier A, van Velthoven R. A comprehensive review of contemporary role of local treatment of the primary tumor and/or the metastases in metastatic prostate cancer. Biomed Res Int. 2014; 2014:501213. PMID: 25485280.

24. Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016; 95:696–702. PMID: 27131082.

25. Clement JM, Sweeney CJ. Evolving treatment of oligometastatic hormone-sensitive prostate cancer. J Oncol Pract. 2017; 13:9–18. PMID: 28045610.

26. Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, Gschwend JE, et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol. 2010; 57:754–761. PMID: 20106588.

27. Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015; 6:8491–8524. PMID: 25940699.

28. Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2013; 2:215. PMID: 23346551.

29. Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013; 11:27–32. PMID: 23010414.

30. Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014; 9:135. PMID: 24920079.

31. Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J Clin Oncol. 2014; 37:227–233. PMID: 22992626.

32. Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016; 69:9–12. PMID: 26189689.

33. Ponti E, Ingrosso G, Carosi A, Di Murro L, Lancia A, Pietrasanta F, et al. Salvage stereotactic body radiotherapy for patients with prostate cancer with isolated lymph node metastasis: a singlecenter experience. Clin Genitourin Cancer. 2015; 13:e279–e284. PMID: 25604915.

34. Schick U, Jorcano S, Nouet P, Rouzaud M, Vees H, Zilli T, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013; 52:1622–1628. PMID: 23544357.

35. Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004; 58:3–10. PMID: 14697414.

36. Tabata K, Niibe Y, Satoh T, Tsumura H, Ikeda M, Minamida S, et al. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulm Med. 2012; 2012:541656. PMID: 22991663.

37. Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018; 73:178–211. PMID: 28655541.
38. Cheng CW, Bergstralh EJ, Zincke H. Stage D1 prostate cancer. A nonrandomized comparison of conservative treatment options versus radical prostatectomy. Cancer. 1993; 71(3 Suppl):996–1004. PMID: 7679047.

39. Frohmüller HG, Theiss M, Manseck A, Wirth MP. Survival and quality of life of patients with stage D1 (T1-3 pN1-2 M0) prostate cancer. Radical prostatectomy plus androgen deprivation versus androgen deprivation alone. Eur Urol. 1995; 27:202–206. PMID: 7601183.
40. Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002; 168:1008–1012. PMID: 12187210.

41. Gakis G, Boorjian SA, Briganti A, Joniau S, Karazanashvili G, Karnes RJ, et al. The role of radical prostatectomy and lymph node dissection in lymph node-positive prostate cancer: a systematic review of the literature. Eur Urol. 2014; 66:191–199. PMID: 23735200.

42. Steuber T, Budäus L, Walz J, Zorn KC, Schlomm T, Chun F, et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int. 2011; 107:1755–1761. PMID: 20942833.

43. Moschini M, Sharma V, Zattoni F, Boorjian SA, Frank I, Gettman MT, et al. Risk stratification of pN+ prostate cancer after radical prostatectomy from a large single institutional series with longterm followup. J Urol. 2016; 195:1773–1778. PMID: 26723866.

44. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006; 7:472–479. PMID: 16750497.

45. Schröder FH, Kurth KH, Fossa SD, Hoekstra W, Karthaus PP, De Prijck L, et al. Early versus delayed endocrine treatment of T2-T3 pN1-3 M0 prostate cancer without local treatment of the primary tumour: final results of European Organisation for the Research and Treatment of Cancer protocol 30846 after 13 years of follow-up (a randomised controlled trial). Eur Urol. 2009; 55:14–22. PMID: 18823693.

46. Sooriakumaran P, Karnes J, Stief C, Copsey B, Montorsi F, Hammerer P, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol. 2016; 69:788–794. PMID: 26038098.

47. Jang WS, Kim MS, Jeong WS, Chang KD, Cho KS, Ham WS, et al. Does robot-assisted radical prostatectomy benefit patients with prostate cancer and bone oligometastases. BJU Int. 2018; 121:225–231. PMID: 28834084.

48. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014; 65:1058–1066. PMID: 24290503.

49. Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol. 2014; 38:435–441. PMID: 24802851.

50. Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol. 2014; 66:602–603. PMID: 24821581.

51. Satkunasivam R, Kim AE, Desai M, Nguyen MM, Quinn DI, Ballas L, et al. Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: a SEER-medicare analysis. J Urol. 2015; 194:378–385. PMID: 25711194.

52. US National Library of Medicine. Local treatment with RP for newly-diagnosed mPCa (LoMP). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02138721.
53. US National Library of Medicine. Cytoreductive prostatectomy in treating patients with newly diagnosed, metastatic prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02458716.
54. Karnes RJ, Murphy CR, Bergstralh EJ, DiMonte G, Cheville JC, Lowe VJ, et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol. 2015; 193:111–116. PMID: 25150640.
55. Jilg CA, Rischke HC, Reske SN, Henne K, Grosu AL, Weber W, et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol. 2012; 188:2190–2197. PMID: 23083862.

56. Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015; 67:299–309. PMID: 24571959.

57. Ploussard G, Almeras C, Briganti A, Giannarini G, Hennequin C, Ost P, et al. Management of node only recurrence after primary local treatment for prostate cancer: a systematic review of the literature. J Urol. 2015; 194:983–988. PMID: 25963190.

58. Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010; 7:44–54. PMID: 19997074.

59. Fowler JF, Toma-Dasu I, Dasu A. Is the α/β ratio for prostate tumours really low and does it vary with the level of risk at diagnosis? Anticancer Res. 2013; 33:1009–1011. PMID: 23482774.
60. Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011; 2011:439752. PMID: 22162711.

61. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012; 84:879–880. PMID: 23078897.

62. Rades D, Veninga T, Stalpers LJ, Basic H, Rudat V, Karstens JH, et al. Outcome after radiotherapy alone for metastatic spinal cord compression in patients with oligometastases. J Clin Oncol. 2007; 25:50–56. PMID: 17194905.

63. Jereczek-Fossa BA, Fariselli L, Beltramo G, Catalano G, Serafini F, Garibaldi C, et al. Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother Oncol. 2009; 93:14–17. PMID: 19409636.

64. Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012; 82:889–897. PMID: 21277113.

65. Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013; 31:455–460. PMID: 21481619.

66. Zagars GK, Pollack A, von Eschenbach AC. Addition of radiation therapy to androgen ablation improves outcome for subclinically node-positive prostate cancer. Urology. 2001; 58:233–239. PMID: 11489709.

67. Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373:301–308. PMID: 19091394.

68. Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011; 378:2104–2111. PMID: 22056152.

69. Lawton CA, Winter K, Grignon D, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node-positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85-31. J Clin Oncol. 2005; 23:800–807. PMID: 15681524.

70. Rusthoven CG, Jones BL, Flaig TW, Crawford ED, Koshy M, Sher DJ, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016; 34:2835–2842. PMID: 27325855.

71. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016; 387:1163–1177. PMID: 26719232.
72. US National Library of Medicine. A phase III of ADT + docetaxel +/− local RT +/− abiraterone acetate in metastatic hormone-naïve prostate cancer. (PEACE1). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01957436.
73. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017; 377:352–360. PMID: 28578607.

74. Mason MD, Clarke NW, James ND, Dearnaley DP, Spears MR, Ritchie AWS, et al. Adding celecoxib with or without zoledronic acid for hormone-naïve prostate cancer: long-term survival results from an adaptive, multiarm, multistage, platform, randomized controlled trial. J Clin Oncol. 2017; 35:1530–1541. PMID: 28300506.

75. Rydzewska LHM, Burdett S, Vale CL, Clarke NW, Fizazi K, Kheoh T, et al. Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur J Cancer. 2017; 84:88–101. PMID: 28800492.
76. Alongi F, Schipani S, Samanes Gajate AM, Rosso A, Cozzarini C, Fiorino C, et al. [11C]choline-PET-guided helical tomotherapy and estramustine in a patient with pelvic-recurrent prostate cancer: local control and toxicity profile after 24 months. Tumori. 2010; 96:613–617. PMID: 20968143.

77. Yao HH, Hong MKh, Corcoran NM, Siva S, Foroudi F. Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia Pac J Clin Oncol. 2014; 10:308–321. PMID: 25155557.

78. Cho SY, Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014; 44:93–109. PMID: 24484747.

79. Minamimoto R, Loening A, Jamali M, Barkhodari A, Mosci C, Jackson T, et al. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J Nucl Med. 2015; 56:1862–1868. PMID: 26405167.

80. Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008; 63:387–395. PMID: 18325358.

81. van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016; 117:732–739. PMID: 26683282.
82. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016; 13:226–235. PMID: 26902337.

83. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68) Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016; 70:926–937. PMID: 27363387.

84. US National Library of Medicine. Non-systemic treatment for patients with low-volume metastatic prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01558427.
85. US National Library of Medicine. Percutaneous high-dose radiotherapy in patients with oligometastases of prostate carcinoma (Oli-P). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02264379.
86. US National Library of Medicine. Best systemic therapy or best systemic therapy (BST) plus definitive treatment (radiation or surgery). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01751438.
87. US National Library of Medicine. Impact of radical prostatectomy as primary treatment in patients with prostate cancer with limited bone metastases (g-RAMPP). accessed on 2018 March 25. Available at: https://clinicaltrials.gov/ct2/show/NCT02454543.
88. Sooriakumaran P. Testing radical prostatectomy in men with prostate cancer and oligometastases to the bone: a randomized controlled feasibility trial. BJU Int. 2017; 120:E8–E20. PMID: 28581205.

89. US National Library of Medicine. Immune responses in prostate, lung, melanoma and breast cancer patients following stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT) or brachytherapy (SBRT). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01777802.
90. US National Library of Medicine. Pembrolizumab and cryosurgery in treating patients with newly diagnosed, oligo-metastatic prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02489357.
91. US National Library of Medicine. Radiosurgery for patients with oligometastatic disease at initial presentation. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01345539.
92. US National Library of Medicine. Radiosurgery for patients recurrent oligometastatic disease. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01345552.
93. US National Library of Medicine. Radiotherapy for oligometastatic prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT01859221.
94. US National Library of Medicine. Phase II study of SBRT as treatment for oligometastases in prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02192788.
95. US National Library of Medicine. Stereotactic radiotherapy for oligometastatic prostate cancer (CROP). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02563691.
96. US National Library of Medicine. Stereotactic body radiation for prostate oligometastases (ORIOLE). accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT02680587.
97. US National Library of Medicine. Intensity-modulated radiation therapy in treating patients undergoing androgen deprivation therapy for metastatic prostate cancer. accessed on 2018 January 10. Available at: https://clinicaltrials.gov/ct2/show/NCT00544830.
Table 1
Definitions of Oligometastatic Prostate Cancer
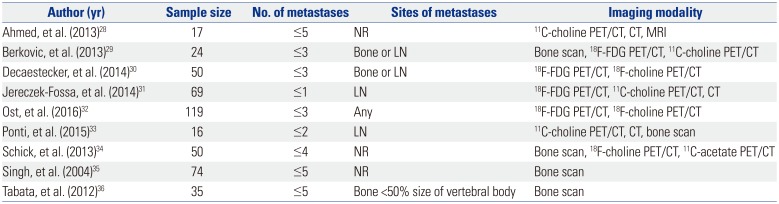
| Author (yr) | Sample size | No. of metastases | Sites of metastases | Imaging modality |
|---|---|---|---|---|
| Ahmed, et al. (2013)28 | 17 | ≤5 | NR | 11C-choline PET/CT, CT, MRI |
| Berkovic, et al. (2013)29 | 24 | ≤3 | Bone or LN | Bone scan, 18F-FDG PET/CT, 11C-choline PET/CT |
| Decaestecker, et al. (2014)30 | 50 | ≤3 | Bone or LN | 18F-FDG PET/CT, 18F-choline PET/CT |
| Jereczek-Fossa, et al. (2014)31 | 69 | ≤1 | LN | 18F-FDG PET/CT, 11C-choline PET/CT, CT |
| Ost, et al. (2016)32 | 119 | ≤3 | Any | 18F-FDG PET/CT, 18F-choline PET/CT |
| Ponti, et al. (2015)33 | 16 | ≤2 | LN | 11C-choline PET/CT, CT, bone scan |
| Schick, et al. (2013)34 | 50 | ≤4 | NR | Bone scan, 18F-choline PET/CT, 11C-acetate PET/CT |
| Singh, et al. (2004)35 | 74 | ≤5 | NR | Bone scan |
| Tabata, et al. (2012)36 | 35 | ≤5 | Bone <50% size of vertebral body | Bone scan |
Table 2
Summary of Studies Using RP or Salvage LN Dissection for Oligometastatic Prostate Cancer
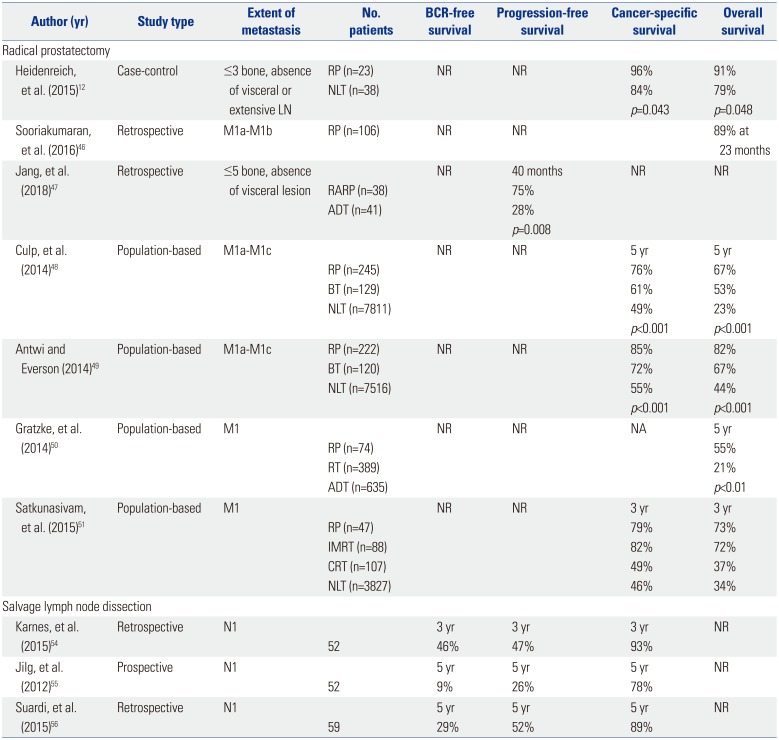
| Author (yr) | Study type | Extent of metastasis | No. patients | BCR-free survival | Progression-free survival | Cancer-specific survival | Overall survival |
|---|---|---|---|---|---|---|---|
| Radical prostatectomy | |||||||
| Heidenreich, et al. (2015)12 | Case-control | ≤3 bone, absence of visceral or extensive LN | RP (n=23) | NR | NR | 96% | 91% |
| NLT (n=38) | 84% | 79% | |||||
| p=0.043 | p=0.048 | ||||||
| Sooriakumaran, et al. (2016)46 | Retrospective | M1a-M1b | RP (n=106) | NR | NR | 89% at 23 months | |
| Jang, et al. (2018)47 | Retrospective | ≤5 bone, absence of visceral lesion | NR | 40 months | NR | NR | |
| RARP (n=38) | 75% | ||||||
| ADT (n=41) | 28% | ||||||
| p=0.008 | |||||||
| Culp, et al. (2014)48 | Population-based | M1a-M1c | NR | NR | 5 yr | 5 yr | |
| RP (n=245) | 76% | 67% | |||||
| BT (n=129) | 61% | 53% | |||||
| NLT (n=7811) | 49% | 23% | |||||
| p<0.001 | p<0.001 | ||||||
| Antwi and Everson (2014)49 | Population-based | M1a-M1c | RP (n=222) | NR | NR | 85% | 82% |
| BT (n=120) | 72% | 67% | |||||
| NLT (n=7516) | 55% | 44% | |||||
| p<0.001 | p<0.001 | ||||||
| Gratzke, et al. (2014)50 | Population-based | M1 | NR | NR | NA | 5 yr | |
| RP (n=74) | 55% | ||||||
| RT (n=389) | 21% | ||||||
| ADT (n=635) | p<0.01 | ||||||
| Satkunasivam, et al. (2015)51 | Population-based | M1 | NR | NR | 3 yr | 3 yr | |
| RP (n=47) | 79% | 73% | |||||
| IMRT (n=88) | 82% | 72% | |||||
| CRT (n=107) | 49% | 37% | |||||
| NLT (n=3827) | 46% | 34% | |||||
| Salvage lymph node dissection | |||||||
| Karnes, et al. (2015)54 | Retrospective | N1 | 3 yr | 3 yr | 3 yr | NR | |
| 52 | 46% | 47% | 93% | ||||
| Jilg, et al. (2012)55 | Prospective | N1 | 5 yr | 5 yr | 5 yr | NR | |
| 52 | 9% | 26% | 78% | ||||
| Suardi, et al. (2015)56 | Retrospective | N1 | 5 yr | 5 yr | 5 yr | NR | |
| 59 | 29% | 52% | 89% | ||||
Table 3
Summary of Studies Using Radiation Therapy as a Metastasis-Directed Therapy for Oligometastatic Prostate Cancer
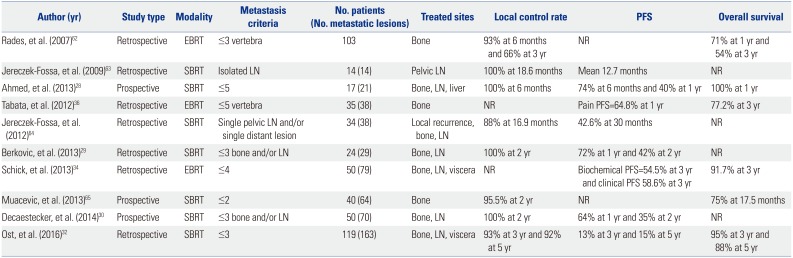
| Author (yr) | Study type | Modality | Metastasis criteria | No. patients (No. metastatic lesions) | Treated sites | Local control rate | PFS | Overall survival |
|---|---|---|---|---|---|---|---|---|
| Rades, et al. (2007)62 | Retrospective | EBRT | ≤3 vertebra | 103 | Bone | 93% at 6 months and 66% at 3 yr | NR | 71% at 1 yr and 54% at 3 yr |
| Jereczek-Fossa, et al. (2009)63 | Retrospective | SBRT | Isolated LN | 14 (14) | Pelvic LN | 100% at 18.6 months | Mean 12.7 months | NR |
| Ahmed, et al. (2013)28 | Prospective | SBRT | ≤5 | 17 (21) | Bone, LN, liver | 100% at 6 months | 74% at 6 months and 40% at 1 yr | 100% at 1 yr |
| Tabata, et al. (2012)36 | Retrospective | EBRT | ≤5 vertebra | 35 (38) | Bone | NR | Pain PFS=64.8% at 1 yr | 77.2% at 3 yr |
| Jereczek-Fossa, et al. (2012)64 | Retrospective | SBRT | Single pelvic LN and/or single distant lesion | 34 (38) | Local recurrence, bone, LN | 88% at 16.9 months | 42.6% at 30 months | NR |
| Berkovic, et al. (2013)29 | Retrospective | SBRT | ≤3 bone and/or LN | 24 (29) | Bone, LN | 100% at 2 yr | 72% at 1 yr and 42% at 2 yr | NR |
| Schick, et al. (2013)34 | Retrospective | EBRT | ≤4 | 50 (79) | Bone, LN, viscera | NR | Biochemical PFS=54.5% at 3 yr and clinical PFS 58.6% at 3 yr | 91.7% at 3 yr |
| Muacevic, et al. (2013)65 | Prospective | SBRT | ≤2 | 40 (64) | Bone | 95.5% at 2 yr | NR | 75% at 17.5 months |
| Decaestecker, et al. (2014)30 | Prospective | SBRT | ≤3 bone and/or LN | 50 (70) | Bone, LN | 100% at 2 yr | 64% at 1 yr and 35% at 2 yr | NR |
| Ost, et al. (2016)32 | Retrospective | SBRT | ≤3 | 119 (163) | Bone, LN, viscera | 93% at 3 yr and 92% at 5 yr | 13% at 3 yr and 15% at 5 yr | 95% at 3 yr and 88% at 5 yr |
Table 4
Summary of Studies Using Radiation Therapy for de novo Oligometastatic Prostate Cancer
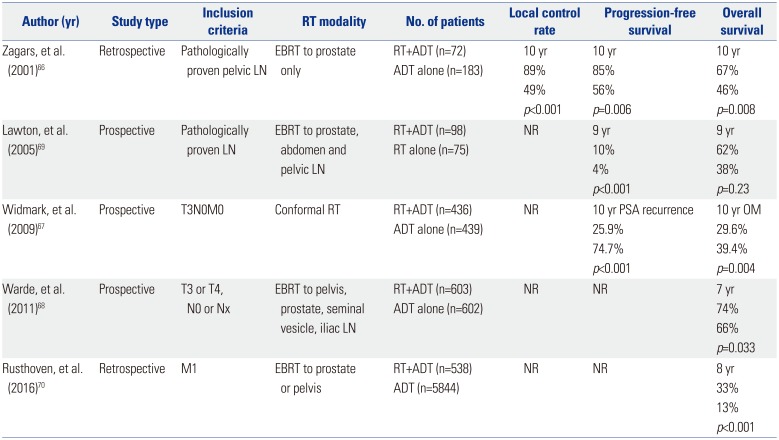
| Author (yr) | Study type | Inclusion criteria | RT modality | No. of patients | Local control rate | Progression-free survival | Overall survival |
|---|---|---|---|---|---|---|---|
| Zagars, et al. (2001)66 | Retrospective | Pathologically proven pelvic LN | EBRT to prostate only | RT+ADT (n=72) | 10 yr | 10 yr | 10 yr |
| ADT alone (n=183) | 89% | 85% | 67% | ||||
| 49% | 56% | 46% | |||||
| p<0.001 | p=0.006 | p=0.008 | |||||
| Lawton, et al. (2005)69 | Prospective | Pathologically proven LN | EBRT to prostate, abdomen and pelvic LN | RT+ADT (n=98) | NR | 9 yr | 9 yr |
| RT alone (n=75) | 10% | 62% | |||||
| 4% | 38% | ||||||
| p<0.001 | p=0.23 | ||||||
| Widmark, et al. (2009)67 | Prospective | T3N0M0 | Conformal RT | RT+ADT (n=436) | NR | 10 yr PSA recurrence | 10 yr OM |
| ADT alone (n=439) | 25.9% | 29.6% | |||||
| 74.7% | 39.4% | ||||||
| p<0.001 | p=0.004 | ||||||
| Warde, et al. (2011)68 | Prospective | T3 or T4, N0 or Nx | EBRT to pelvis, prostate, seminal vesicle, iliac LN | RT+ADT (n=603) | NR | NR | 7 yr |
| ADT alone (n=602) | 74% | ||||||
| 66% | |||||||
| p=0.033 | |||||||
| Rusthoven, et al. (2016)70 | Retrospective | M1 | EBRT to prostate or pelvis | RT+ADT (n=538) | NR | NR | 8 yr |
| ADT (n=5844) | 33% | ||||||
| 13% | |||||||
| p<0.001 |
Table 5
Summary of Clinical Trials Investigating Treatment of Oligometastatic Prostate Cancer
| Identifier | Trial title | Number of metastasis | Sites of metastasis |
|---|---|---|---|
| Surgery | |||
| NCT0213872152 | Local treatment with radical prostatectomy for newly-diagnosed metastatic prostate cancer | NR | N1, M1 |
| NCT0245871653 | Feasibility of cytoreductive prostatectomy in men newly diagnosed with metastatic prostate cancer | NR | N1, M1 |
| SRCTN1570486288 | Testing radical prostatectomy in men with prostate cancer and oligometastases to the bone: a randomized controlled feasibility trial (TRoMbone) | ≤3 | Bone |
| NCT0245454387 | Multicenter, prospective, randomized controlled trial comparing radical prostatectomy plus best systemic therapy with best systemic therapy alone in the management of men with pauci-metastatic prostate cancer | ≤5 | Bone, LN |
| Radiation therapy | |||
| NCT0134553991 | Phase II study for curative intent treatment for patients with oligometastatic disease at initial presentation | ≤5 | NR |
| NCT0134555292 | Phase II study of stereotactic radiosurgery for patients with oligo-recurrent disease | ≤5 | NR |
| NCT0177780289 | Observational study of immune responses in prostate, lung, melanoma and breast cancer patients following stereotactic body radiotherapy, intensity modulated radiotherapy, or brachytherapy | ≤3 | NR |
| NCT0185922193 | Phase II stereotactic body radiotherapy and stereotactic hypofractionated radiotherapy for oligometastatic prostate cancer | NR | Any except brain or CNS |
| NCT0219278894 | Phase II study of SBRT as treatment for oligometastases in prostate cancer | ≤4 | Bone, LN |
| NCT0226437985 | Effectiveness and toxicity of a percutaneous high-dose radiotherapy in patients with oligometastases of prostate carcinoma | ≤5 | NR |
| NCT0256369195 | Comprehensive stereotactic radiotherapy for oligometastatic prostate cancer: A Phase I/II study | ≤5 | Outside prostate and pelvic LN |
| NCT0268058796 | Phase II randomized observation versus stereotactic ablative radiation for oligometastatic prostate cancer (ORIOLE) trial | ≤3 | Bone, LN |
| Systemic therapy/combination therapy | |||
| NCT0026847674 | STAMPEDE: Systemic therapy in advanced or metastatic prostate cancer: Evaluation of drug efficacy - androgen suppression-based therapy alone or combined with zoledronic acid, docetaxel, prednisolone, celecoxib, abiraterone, enzalutamide and/or radiotherapy, metformin and transdermal estradiol in treating patients with locally advanced or metastatic prostate cancer | NR | N1, M1 |
| NCT0054483097 | Androgen deprivation and localized radiotherapy to metastases in patients with oligometastatic hormone-sensitive prostate cancer | ≤5 | N1, M1 |
| NCT0155842784 | Salvage treatment or active clinical surveillance for oligometastatic prostate cancer: a randomized phase II trial | ≤3 | N1, M1a/b |
| NCT0175143886 | A prospective, multi-institutional, randomized, phase II trial of best systemic therapy or best systemic therapy plus definitive treatment (radiation or surgery) of the primary tumor in metastatic (M1) prostate cancer | NR | Any except brain |
| NCT0195743672 | A phase III of ADT +/− docetaxel +/− local RT +/− abiraterone acetate in metastatic hormonenaïve prostate cancer | NR | Outside pelvic LN |
| NCT0248935790 | A pilot study of MK-3475 with cryotherapy for men with newly diagnosed oligo-metastatic prostate cancer | ≤4 | Extrapelvis |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download