Abstract
Objective
To characterize the hematologic profile of preterm fetuses delivered spontaneously with acute histologic chorioamnionitis (HCA).
Methods
This was a retrospective cohort study. The relationship between the presence of acute HCA and the change of hematologic profile was examined in 109 singleton pregnant women who were admitted and delivered between 24-32 weeks of gestation. Cases without results of placental histologic examination, cord blood cell count, and the differential count were excluded. From the cord blood, hemoglobin concentration, hematocrit, mean corpuscular volume, total leucocyte count and the differential count, platelet count, normoblast count, and umbilical arterial pH were obtained. All the observed values were corrected for gestational age by calculating a ratio between the observed and mean expected value for gestational age.
Results
1) The prevalence of acute HCA was 60.6% (66/109); 2) newborns with acute HCA had a higher median corrected leucocyte counts and corrected percentage of neutrophil in the differential count and a lower median corrected percentage of lymphocyte in the differential count than those without acute HCA; 3) neutrophilia was significantly frequent in newborns with acute HCA than in those without acute HCA; and 4) acute HCA was not associated with detectable changes in percentage of monocyte, eosinophil, basophil, and normoblast in the differential count, hemoglobin concentration, hematocrit, erythrocyte counts, mean corpuscular volume, platelet counts, or umbilical arterial pH.
REFERENCES
1). Yoon BH., Romero R., Kim CJ., Jun JK., Gomez R., Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995. 172:960–70.

2). Chang JW., Yoon BH., Syn HC. The relationship between the presence, severity and pattern of acute placental inflammation and amniotic fluid interleukin-8 in preterm labor. Korean J Obstet Gynecol. 1999. 42:2669–74.
3). Kim JC., Yoon BH. The relationship between amniotic fluid white blood cell count and the presence and severity of acute placental inflammation in preterm premature rupture of membrane. Korean J Obstet Gynecol. 2000. 43:885–90.
4). Murphy DJ., Sellers S., MacKenzie IZ., Yudkin PL., Johnson AM. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995. 346:1449–54.

5). Watterberg KL., Demers LM., Scott SM., Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996. 97:210–5.

6). Lee JS., Choi SJ., Moon SO., Yang SH., Lee KS. The association of histologic chorioamnionitis and bronchopulmonary dysplasia in prematurity. Korean J Obstet Gynecol. 2002. 45:1478–84.
7). Park KH., Yoon BH., Jun JK., Park JS., Kim GJ., Lee HK, et al. The relationship between amniotic fluid tumor necrosis factor-, histologic chorioamnionitis, and congenital sepsis in preterm labor. Korean J Obstet Gynecol. 2001. 44:946–56.
8). Gomez R., Romero R., Ghezzi F., Yoon BH., Mazor M., Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998. 179:194.

9). Fung YL., Fraser JF., Wood P., Minchinton RM., Silliman CC. The systemic inflammatory response syndrome induces functional changes and relative hyporesponsiveness in neutrophils. J Crit Care. 2008. 23:542–9.

10). Groselj-Grenc M., Ihan A., Pavcnik-Arnol M., Kopitar AN., Gmeiner-Stopar T., Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009. 35:1950–8.

11). Grimm RH Jr., Neaton JD., Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985. 254:1932–7.

12). Mammen EF. The haematological manifestations of sepsis. J Antimicrob Chemother. 1998. 41(Suppl A):17–24.

13). Koussoulas V., Tzivras M., Karagianni V., Spyridaki E., Plachouras D., Giama-rellou H, et al. Monocytes in systematic inflammatory response syndrome: differences between sepsis and acute pancreatitis. World J Gastroenterol. 2006. 12:6711–4.

14). Stachon A., Segbers E., Holland-Letz T., Kempf R., Hering S., Krieg M. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care. 2007. 11:R62.

15). Nupponen I., Pesonen E., Andersson S., Mäkelä A., Turunen R., Kautiainen H, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002. 110(1 Pt 1):36–41.

16). Papoff P., Christensen RD., Calhoun DA., Juul SE. Granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor and neutrophils in the bronchoalveolar lavage fluid of premature infants with respiratory distress syndrome. Biol Neonate. 2001. 80:133–41.

17). Schrama AJ., de Beaufort AJ., Poorthuis BJ., Berger HM., Walther FJ. Secretory phospholipase A(2) in newborn infants with sepsis. J Perinatol. 2008. 28:291–6.

18). Selimović A., Skokić F., Selimović Z., Bazardzanović M. The predictive values of total white blood count and differential count in the diagnosis of early-onset neonatal sepsis. Med Arh. 2008. 62:205–10.
19). Wirbelauer J., Thomas W., Speer CP. Response of leukocytes and nucleated red blood cells in very low-birth weight preterm infants after exposure to intrauterine inflammation. J Matern Fetal Neonatal Med. 2011. 24:348–53.

20). Romero R., Savasan ZA., Chaiworapongsa T., Berry SM., Kusanovic JP., Hassan SS, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011. 40:19–32.

21). Romero R., Soto E., Chaiworapongsa T., Berry SM., Hassan SS., Kusanovic JP, et al. Blood pH and gases in fetuses in preterm labor with and without a systemic inflammatory response syndrome. J Matern Fetal Neonatal Med. 2012. 25:1160–70.
22). Kang MS., Kim YH., Kim CH., Kim KM., Cho MK., Kim JW, et al. Changes of interleukin-6, C-reactive protein, and lipid peroxide levels in the umbilical venous plasma of preterm birth with or without chorioamnionitis. Korean J Perinatol. 2007. 18:352–61.
23). Kim EN., Kim CJ., Park JW., Yoon BH. Acute funisitis is associated with distinct changes in fetal hematologic profile. J Matern Fetal Neonatal Med. 2015. 28:588–93.

24). Pacora P., Chaiworapongsa T., Maymon E., Kim YM., Gomez R., Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002. 11:18–25.

25). Kim CJ., Yoon BH., Park SS., Kim MH., Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001. 32:623–9.

26). Kim CJ., Romero R., Chaemsaithong P., Chaiyasit N., Yoon BH., Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015. 213(4 Suppl):S29–52.

27). Davies NP., Buggins AG., Snijders RJ., Jenkins E., Layton DM., Nicolaides KH. Blood leucocyte count in the human fetus. Arch Dis Child. 1992. 67(4 Spec No):399–403.

28). Nicolaides KH., Thilaganathan B., Mibashan RS. Cordocentesis in the investigation of fetal erythropoiesis. Am J Obstet Gynecol. 1989. 161:1197–200.

29). Forestier F., Daffos F., Catherine N., Renard M., Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood. 1991. 77:2360–3.

30). Nicolaides KH., Economides DL., Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989. 161:996–1001.

31). De Waele M., Foulon W., Renmans W., Segers E., Smet L., Jochmans K, et al. Hematologic values and lymphocyte subsets in fetal blood. Am J Clin Pathol. 1988. 89:742–6.

32). Kim SK., Romero R., Chaiworapongsa T., Kusanovic JP., Mazaki-Tovi S., Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009. 37:543–52.

33). Leikin E., Garry D., Visintainer P., Verma U., Tejani N. Correlation of neonatal nucleated red blood cell counts in preterm infants with histologic chorioamnionitis. Am J Obstet Gynecol. 1997. 177:27–30.

34). de Alarcón PA., Johnson MC., Werner EJ. Neonatal hematology, in Erythropoiesis, red cells, and the approach to anemia, edited by de Alarcón PA, Werner EJ, eds. 4th ed. New York, Cambridge University Press, 2005, p. 40–57.
35). Oakes M., MacDonald H., Wilson D. Abnormal laboratory test values during ceftriaxone therapy. Am J Med. 1984. 77:89–96.
36). Moskovitz BL. Clinical adverse effects during ceftriaxone therapy. Am J Med. 1984. 77:84–8.
37). Russell P. Inflammatory lesions of the human placenta. I. clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet. 1979. 1:127–37.
Fig. 1.
Comparison of the fetal blood absolute and corrected leucocyte counts. (A) Fetuses with acute histologic chorioamnionitis had higher median absolute leucocyte counts than those without acute histologic chorioamnionitis (absolute leucocyte count median 10.5 [range, 1.1-35.4] vs. 6.6 [range, 2.4-15.9] ×109/L, P<0.001). (B) Corrected leucocyte counts by gestational age was also higher in fetuses with acute histologic chorioamnionitis (corrected leucocyte count median 2.31 [range, 0.27-8.68] vs. 1.11 [range, 0.41-3.97], P<0.001). HCA, histologic chorioamnionitis.
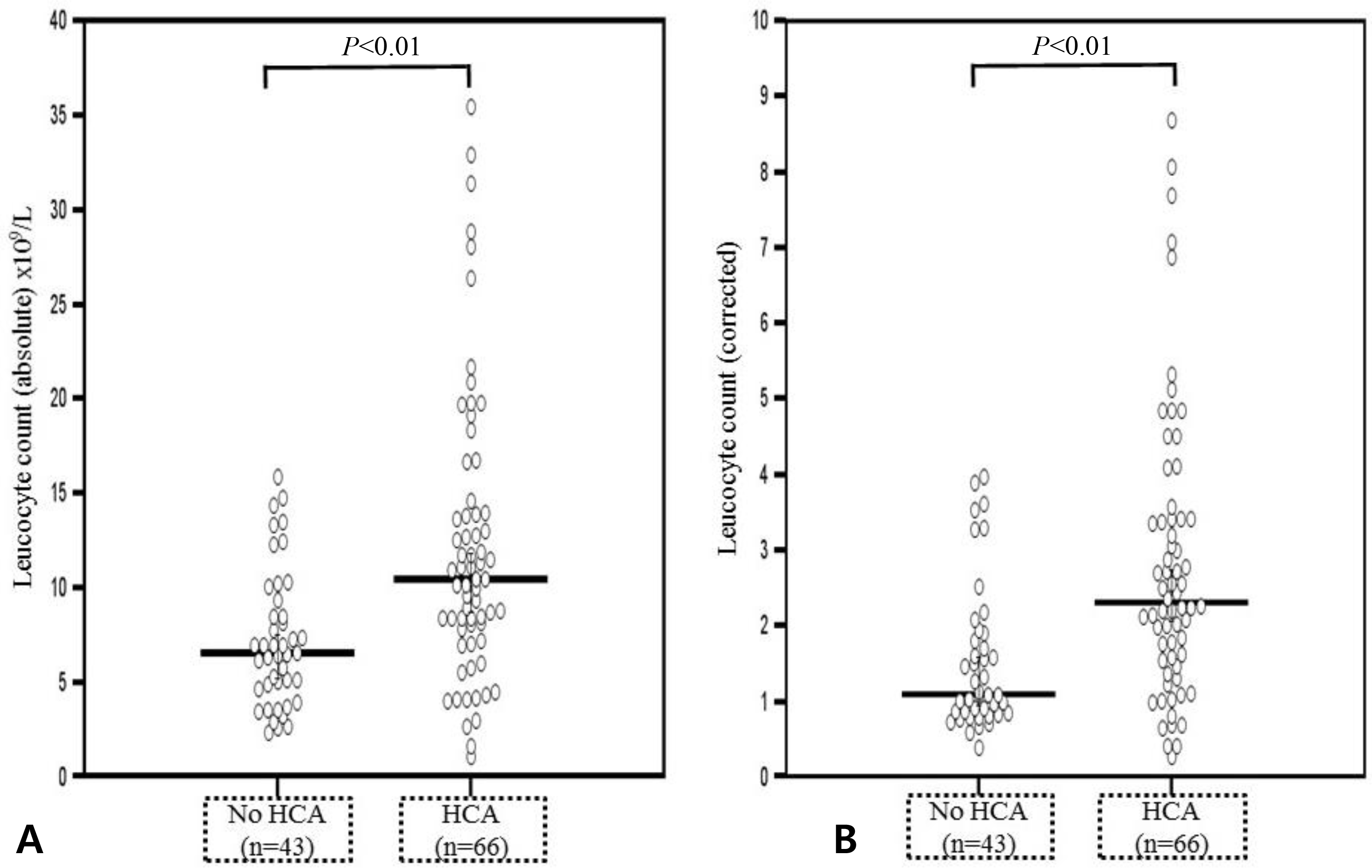
Fig. 2.
Comparison of the fetal blood percentage of neutrophil in the differential count and corrected neutrophil counts. (A) Median percentage of neutrophil was higher in fetuses with acute histologic chorioamnionitis than in those without acute histologic chorioamnionitis (median percentage of neutrophil in the differential count 53.5% [range, 5.0-85.1] vs. 32.5% [range, 5.0-71.9], P<0.001). (B) Corrected median percentage of neutrophil counts was also higher in fetuses with acute histologic chorioamnionitis (corrected median percentage of neutrophil counts 5.51 [range, 0.43-11.2] vs. 2.20, [range 0.26-5.75], P<0.001). HCA, histologic chorioamnionitis.
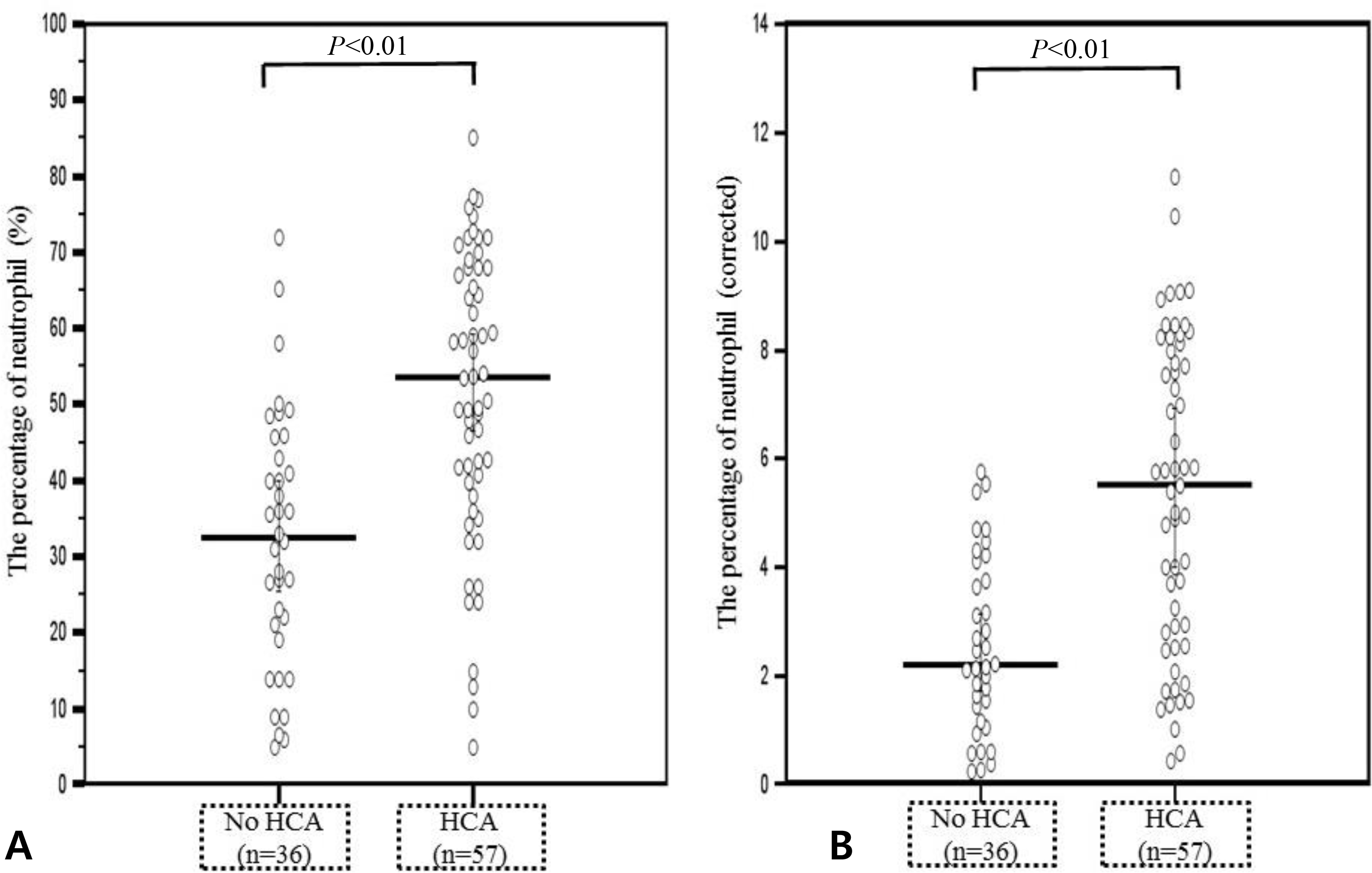
Fig. 3.
Comparison of the fetal blood percentage of lymphocyte in the differential count. (A) Median percentage of lymphocyte was lower in fetuses with acute histologic chorioamnionitis than those without acute histologic chorioamnionitis (median percentage of lymphocyte in the differential count 32.4% [range, 8.3-86.0] vs. 53.0% [range, 18.1-85.0], P<0.001). (B) Corrected percentage of lymphocyte counts was also lower in fetuses with acute histologic chorioamnionitis (corrected median percentage of lymphocyte counts 0.41 [range, 0.12-1.26] vs. 0.69 [range, 0.26-1.24], P<0.001). HCA, histologic chorioamnionitis
Table 1.
Demographic and Clinical Characteristics of the Study Population according to the Presence or Absence of Acute Histologic Chorioamnionitis
| Acute histologic chorioamnionitis | P-value | ||
|---|---|---|---|
| Absent (n=43) | Present (n=66) | ||
| Maternal age (yrs) | 30.2 (22.0-45.0) | 31.5 (25.0-43.0) | 0.314 |
| Nulliparity | 24/43 (55.8) | 28/66 (42.4) | 0.171 |
| Etiology of preterm birth | 0.167 | ||
| Preterm labor | 28/43 (65.1) | 33/66 (50.0) | |
| PROM | 15/43 (34.9) | 33/66 (50.0) | |
| Cesarean delivery | 26/43 (60.5) | 28/66 (42.4) | 0.066 |
| Antenatal corticosteroids use | 32/43 (74.4) | 52/66 (78.8) | 0.596 |
| Antenatal antibiotics use | 22/43 (51.2) | 53/66 (80.3) | 0.001 |
| Tocolytics use | 34/43 (79.1) | 46/66 (69.7) | 0.279 |
| Clinical chorioamnionitis | 1/43 (2.3) | 4/66 (6.1) | 0.646 |
| Gestational age at delivery (wks) | 29.7 (24.0-32.0) | 28.2 (24.1-32.0) | 0.001 |
| Birthweight (g) | 1,410.0 (710.0-2,610.0) | 1,150.0 (530.0-2,020.0) | 0.008 |
| AF WBC count ≥20 cells/mm3∗ | 8/23 (34.8) | 32/51 (62.7) | 0.025 |
| Positive AF culture∗ | 3/23 (13.0) | 12/51 (23.5) | 0.365 |
Values are presented as median (range) or number (%). Abbreviations: PROM, premature rupture of membranes; AF, amniotic fluid; WBC, white blood cell.
∗ Transabdominal amniocentesis was performed only in 40 women from whom we received a written consent. In women without acute histologic chorioamnionitis, 61% (14/23) were diagnosed as having preterm labor. Fifty three percent (27/51) had preterm labor in women with acute histologic chorioamnionitis.
Table 2.
Hematologic Profile of the Fetuses according to the Presence or Absence of Acute Histologic Chorioamnionitis
| Acute histologic chorioamnionitis | P-value | ||
|---|---|---|---|
| Absent (n=43) | Present (n=66) | P-value | |
| Leucocyte (×109/L) | 6.6 (2.4-15.9) | 10.5 (1.1-35.4) | <0.001 |
| Corrected leucocyte | 1.11 (0.41-3.97) | 2.31 (0.27-8.68) | <0.001 |
| Neutrophilia | 25/36 (69.4)† | 52/57 (91.2)‡ | 0.007 |
| Neutropenia | 1/36 (92.8)† | 0/57 (0.0)‡ | 0.387 |
| Percentage of neutrophil (%)∗ | 32.5 (5.0-71.9) | 53.5 (5.0-85.1) | <0.001 |
| Corrected percentage of neutrophil∗ | 2.20 (0.26-5.75) | 5.51 (0.43-11.2) | <0.001 |
| Percentage of lymphocyte (%)∗ | 53.0 (18.1-85.0) | 32.4 (8.3-86.0) | <0.001 |
| Corrected percentage of lymphocyte∗ | 0.69 (0.26-1.24) | 0.41 (0.12-1.26) | <0.001 |
| Percentage of monocyte (%)∗ | 7.2 (2.0-19.0)† | 7.1 (0.0-24.0)‡ | 0.981 |
| Corrected percentage of monocyte∗ | 2.38 (0.67-6.33)† | 2.37 (0.0-8.0)‡ | 0.981 |
| Percentage of eosinophil (%)∗ | 2.6 (0.0-11.0)† | 1.6 (0.0-9.0)‡ | 0.057 |
| Percentage of basophil (%)∗ | 0.1 (0.0-3.4)† | 0.17 (0.0-6.2)‡ | 0.976 |
| Nucleated RBC (/100WBCs) | 10.5 (0.0-237.0)† | 0.9 (0.0-175.0)‡ | 0.114 |
| Corrected nucleated RBC | 0.58 (0.0-12.2)† | 0.04 (0.0-8.33)‡ | 0.098 |
| Erythrocyte (×1012/L) | 3.60 (2.25-4.56) | 3.42 (1.60-5.37) | 0.059 |
| Corrected erythrocyte | 0.99 (0.65-1.31) | 0.97 (0.48-1.41) | 0.217 |
| Hemoglobin (g/dL) | 13.6 (2.4-18.1) | 12.8 (6.0-20.6) | 0.085 |
| Corrected hemoglobin | 1.04 (0.19-1.33) | 0.98 (0.46-1.51) | 0.176 |
| Hematocrit (%) | 41.5 (23.9-54.0) | 39.8 (16.2-61.5) | 0.229 |
| Corrected hematocrit | 1.00 (0.58-1.30) | 0.95 (0.40-1.41) | 0.401 |
| MCV (×10-15/L) | 111.9 (92.1-127.3) | 114.9 (96.4-146.4) | 0.173 |
| Corrected MCV | 0.96 (0.78-1.11) | 0.97 (0.10-1.21) | 0.507 |
| Platelet (×109/L) | 206.0 (16.0-380.0) | 232.5 (7.0-491.0) | 0.190 |
| Corrected platelet | 0.88 (0.06-1.57) | 0.98 (0.03-2.03) | 0.224 |
| Umbilical arterial pH | 7.29 (6.85-7.43)§ | 7.30 (6.91-7.53)II | 0.593 |
| Corrected umbilical arterial pH | 0.99 (0.93-1.00)§ | 0.99 (0.92-1.00)II | 0.480 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download