Abstract
Choriocarcinoma is a rare malignant germ cell tumor and it usually occurs in the gonads (ovary or testis) and uterus. Primary hepatic choriocarcinoma (PHC) is a variant of choriocarcinoma featuring sole liver presentation without any evidence of gonodal involvements. Adult male patients with PHC carry dismal prognosis and their median survival period was less than 5 months. We herein present a first Korean case of a 54-year-old male patient with adult PHC, who was treated by surgical resection and chemotherapy through a multidisciplinary approach.
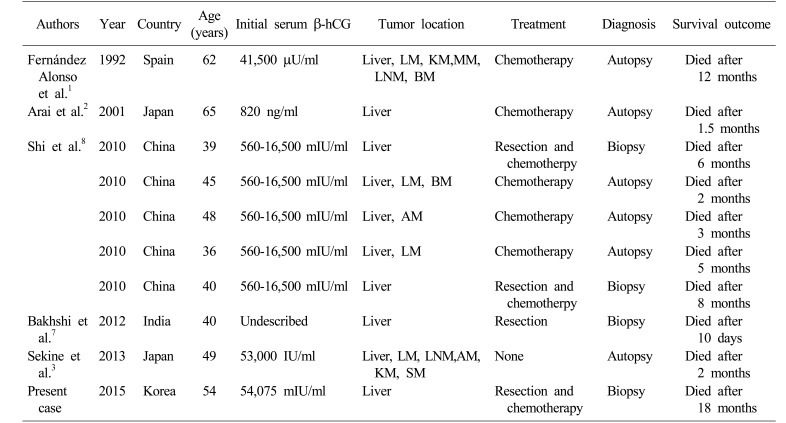
Choriocarcinoma is one of the germ cell tumors composed exclusively of syncytioblastic and cytotrophoblastic cells.1 Primary hepatic choriocarcinoma (PHC) is characterized by liver involvement without a detectable primary lesion in gonads, retroperitoneum, mediastinum, bladder or prostate.2 It is extremely rare but highly aggressive germ cell tumor subtype that tends to disseminate even in an early stage.3 To date, there exist only 5 case reports involving 9 adult male patients and most of the patients were diagnosed only after tumor rupture or distant metastasis (Table 1). Herein, we report the first case of an adult male patient with PHC in Korea who was managed by palliative resection and subsequent systemic chemotherapy.
A 54-year-old man, a known hepatitis B carrier, and heavy alcoholic, was referred to our institution with a liver mass on routine abdominal ultrasonography. We performed physical examination, laboratory tests, and imaging workup. He had no abdominal pain on palpation and the initial tumor markers were in normal level: α-fetoprotein (AFP) 3.4 IU/ml (normal range: 0–5 IU/ml), proteins induced by vitamin K deficiency or antagonists-II (PIVKA-II) 17 mAU/ml (normal range: 0–40 mAU/ml), Carbohydrate antigen (CA) 19-9 7.4 U/ml (normal range: 0–37 U/ml), and chorioembryonic antigen (CEA) 2.6 ng/ml (normal range: 0–6 ng/ml). Dynamic abdomen computed tomography (CT) scan (Fig. 1A) and magnetic resonance imaging of the liver showed the presence of a 6.3-cm sized exophytic mass in the segment IV of the liver. The imaging revealed peripheral wall enhancement and internal hemorrhage.
We suspected that it could be an atypical hepatocellular carcinoma or intrahepatic cholangiocarcinoma, thus ultrasonography-guided liver biopsy was conducted. The pathologic report revealed poorly differentiated carcinoma in a massive necrotic background. The results of immunohistochemical staining were CK 19(+), CK 7(+), CK 20(+), hepatocyte(−) and glypican 3(+), but it was difficult to reach a final diagnosis because the viable tissue component was very small (less than 5%). A multidisciplinary discussion was held with surgeons, physicians and pathologists, and the patient was recommended to primarily receive hepatic tumor resection.
Three weeks after liver biopsy, he was admitted for surgery. The indocyanine green retention rate at 15 minutes (ICG R15) was 23.1%. Preoperative chest CT scan (Fig. 1B–D) showed the absence of distant metastasis, but increase in size of the primary hepatic lesion (11 cm) and another four small (1–1.5 cm) masses in both the hepatic lobes. Considering high ICG R15 value and large left hepatic volume (43% of the total liver volume) on cirrhotic background liver, a palliative resection with left medial sectionectomy was performed to confirm the diagnosis as well as to reduce the tumor burden. Liver contour was in early cirrhotic phase with nodularity and the main mass was firmly attached to the greater omentum in the operative findings. Palliative left medial sectionectomy was performed, but we could not resect the other nodules because they were neither localizable nor palpable. The operation was carried out for 6 hours and the total bleeding amount was 1,235 ml. Since his vital signs were stable, no further transfusions were given.
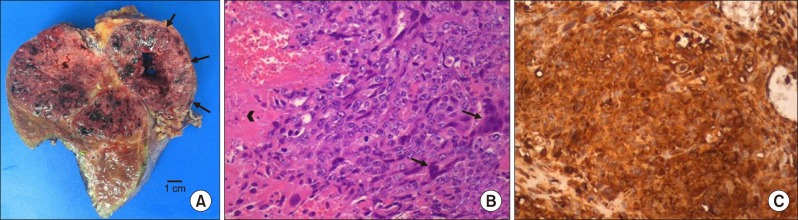
After one week, choriocarcinoma was pathologically confirmed, so we checked β-human chorionic gonadotropin (hCG) subunit level (54,075 mIU/ml, normal range: 0–10 mIU/ml) and bilateral scrotal ultrasonography showed no abnormal findings. One week later, remnant tumors increased from 1–1.5 cm to 2–2.5 cm on postoperative CT scan (Fig. 1E, F). The patient was recommended to receive systemic chemotherapy after multidisciplinary tumor board discussion. Immunohistochemical stains showed CK 19(+), CK 7(+), MOC-31(+), hepatocyte(−), glypican 3(+), cluster of differentiation (CD) 31(+), AFP(−), and β-hCG(+, diffuse). Gross appearance of the tumor was a multi-lobulated hemorrhagic mass, measuring 11.1×8.5×8.5 cm in size. It extended to the extracapsular portion with adhesion to the omentum. Microscopically, it consisted of a biphasic proliferation of mononuclear cytotrophoblasts and multinucleated syncytiotrophoblasts (Fig. 2). Cellular pleomorphism, brisk mitotic activity, extensive necrosis, and hemorrhage were observed.
Three weeks after surgery, the medical oncologists initiated the standard chemotherapy regimen of EMA-CO (Etoposide on day 1–2, Methotrexate on day 1, Dactinomycin on day 1–2, Cyclophosphamide on day 8, and Vincristine on day 8). Four cycles of chemotherapy were given without significant adverse events and the patient achieved partial response (PR) based on Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1)4 on imaging follow-up (Fig. 1G, H). Serial β-hCG levels were checked at the beginning of each cycle. The level rapidly went down to 1,003 mIU/ml at the fourth cycle but did not decrease further afterward. Subsequently, we planned to perform curative resection if the remnant lesions exhibited responsiveness, after team discussions.
But the next follow up CT after the seventh cycle revealed progressive disease (PD) by RECIST. At this time, we decided to switch to another palliative regimen called EMA-EP (Etoposide on day 1 and 8, Methotrexate on day 1, Dactinomycin on day 1–2, and Cisplatin on D8). After two cycles, the patient achieved stable disease (SD) by RECIST and the β-hCG level dropped down to 269 mIU/ml. The patient was alive for 10 months after initial surgery and was on the fourth EMA-EP cycle in a good performance state. Despite nine cycles of chemotherapy, hepatic metastases progressed rapidly. Finally, he died due to hepatic failure subsequent to only 18 months after initial surgery.
Choriocarcinoma comprises only 1% of all germ cell neoplasms and is more common in women.56 It is a β-hCG secreting tumor which is pertinent to uterus and pregnancy.7 PHC is defined as a choriocarcinoma in the liver, which excludes metastasis from another primary origin, especially from the testis in men.8 The majority of PHC is an infantile subtype, which represents metastasis from occult placental choriocarcinoma. Pathogenesis of adult PHC is not clearly understood, but the tumor is assumed to arise from abnormal migration of germ cells during embryogenesis or from different histogenetic origin.9
So far, only 9 adult male patients with PHC have been presented in the last 20 years in English literature (Table 1),12378 and a majority of them (8 patients) were Asian. Their mean age at the time of diagnosis was 47.6 years (ranging from 36 to 65 years), and initial serum β-hCG level was elevated except in an undescribed case. Primary hepatic involvement was observed in half of the patients but the other half had disseminated disease at diagnosis or on autopsy. Since PHC grows rapidly, 6 patients were diagnosed by postmortem inspection, usually in ruptured or metastasized state. Only 4 patients were able to undergo curative surgical resection.
The overall prognosis of PHC in adult male patients had been consistently poor with a median survival period of 5 months (ranging from 10 days to 12 months). And owing to its paucity of incidence, there have been no optimal diagnostic and therapeutic strategies to improve patient outcomes. In our case, we could not anticipate PHC at first and our liver biopsy yielded no diagnostic clues. Only after surgical resection, we reached a final diagnosis and checked serum β-hCG level. In men in their 40's or in 50's with atypical and quickly growing hepatic lesions, it can be difficult to distinguish hepatocellular carcinoma with sarcomatous change or cholangiocarcinoma from PHC. Since cytotrophoblasts are histologically similar to poorly-differentiated carcinoma, diagnostic accuracy of liver biopsy can be unsatisfactory. However, Sekine et al.3 emphasized that liver biopsy should be performed without hesitation in order to enable earlier treatment of such an aggressive tumor. We presume that liver biopsy for earlier histological confirmation is still important and it can be also helpful to investigate the changes in the levels of serum β-hCG as well as other tumor markers such as AFP, CA 19-9, and CEA, especially when the tumor is rapidly progressive in middle-aged men. Employment of immunohistochemical battery of antibodies against CK, β-hCG, HPL and Ki-67 when reviewing pathologic slides is also of great importance.8
The role of chemotherapy in the treatment of PHC is unclear due to the tumor's rapid clinical deterioration, but some authors reported a pathologic complete response and 2-year disease-free survival with etoposide and cisplatin in gastric choriocarcinoma.10 Cisplatin and 5-fluorouracil combination has been challenged as adjuvant or palliative chemotherapy but the clinical outcome was not satisfactory as per another published report.8 We applied EMA-CO and EMA-EP regimens to our patient. These regimens are widely used in female gestational trophoblastic tumor patients and have demonstrated clinical efficacy with moderate toxicities.
PHC in adult male patients is known to be a very aggressive subset of germ cell tumors and is commonly found in the Asian population. Because of its highly malignant potential, tumor proliferates and disseminates rapidly. In middle-aged men with atypically and rapidly deteriorating hepatic lesions, we should suspect PHC as a differential diagnosis of hepatic tumors. When the pathologic finding of liver biopsy is uncertain, checking additional serum β-hCG level can be of importance since delayed diagnosis of PHC can be fatal.
References
1. Fernández Alonso J, Sáez C, Pérez P, Montaño A, Japón MA. Primary pure choriocarcinoma of the liver. Pathol Res Pract. 1992; 188:375–377. discussion 378–379. PMID: 1626001.

2. Arai M, Oka K, Nihei T, Hirota K, Kawano H, Kawasaki T, et al. Primary hepatic choriocarcinoma: a case report. Hepatogastroenterology. 2001; 48:424–426. PMID: 11379323.
3. Sekine R, Hyodo M, Kojima M, Meguro Y, Suzuki A, Yokoyama T, et al. Primary hepatic choriocarcinoma in a 49-year-old man: report of a case. World J Gastroenterol. 2013; 19:9485–9489. PMID: 24409080.

4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.

5. Scholz M, Zehender M, Thalmann GN, Borner M, Thoni H, Studer UE. Extragonadal retroperitoneal germ cell tumor: evidence of origin in the testis. Ann Oncol. 2002; 13:121–124. PMID: 11863093.

6. Fine G, Smith RW Jr, Pachter MR. Primary extragenital choriocarcinoma in the male subject. Case report and review of the literature. Am J Med. 1962; 32:776–794. PMID: 13892927.
7. Bakhshi GD, Borisa AD, Bhandarwar AH, Tayade MB, Yadav RB, Jadhav YR. Primary hepatic choriocarcinoma: a rare cause of spontaneous haemoperitoneum in an adult. Clin Pract. 2012; 2:e73. PMID: 24765472.

8. Shi H, Cao D, Wei L, Sun L, Guo A. Primary choriocarcinoma of the liver: a clinicopathological study of five cases in males. Virchows Arch. 2010; 456:65–70. PMID: 20013345.

9. Garcia RL, Ghali VS. Gastric choriocarcinoma and yolk sac tumor in a man: observations about its possible origin. Hum Pathol. 1985; 16:955–958. PMID: 4040885.

10. Waseda Y, Komai Y, Yano A, Fujii Y, Noguchi N, Kihara K. Pathological complete response and two-year disease-free survival in a primary gastric choriocarcinoma patient with advanced liver metastases treated with germ cell tumor-based chemotherapy: a case report. Jpn J Clin Oncol. 2012; 42:1197–1201. PMID: 23071288.

Fig. 1
Pre- and postoperative computed tomography (CT) scan findings. Preoperative CT scan showed that the initial tumor diameter was 6.3 cm (A). The size of the main tumor increased to 11 cm after 3 weeks (B), and new nodules (arrow) were found on the right and left lobes in the same CT scan (C and D); CT scan taken 1 week after surgery showed increase in the sizes of the remnant tumors (arrows) to up to 2.5 cm (E and F); Follow-up CT after chemotherapy showed a decrease in the size of the remnant multiple tumors (arrows) to achieve partial response by RECIST (G and H).
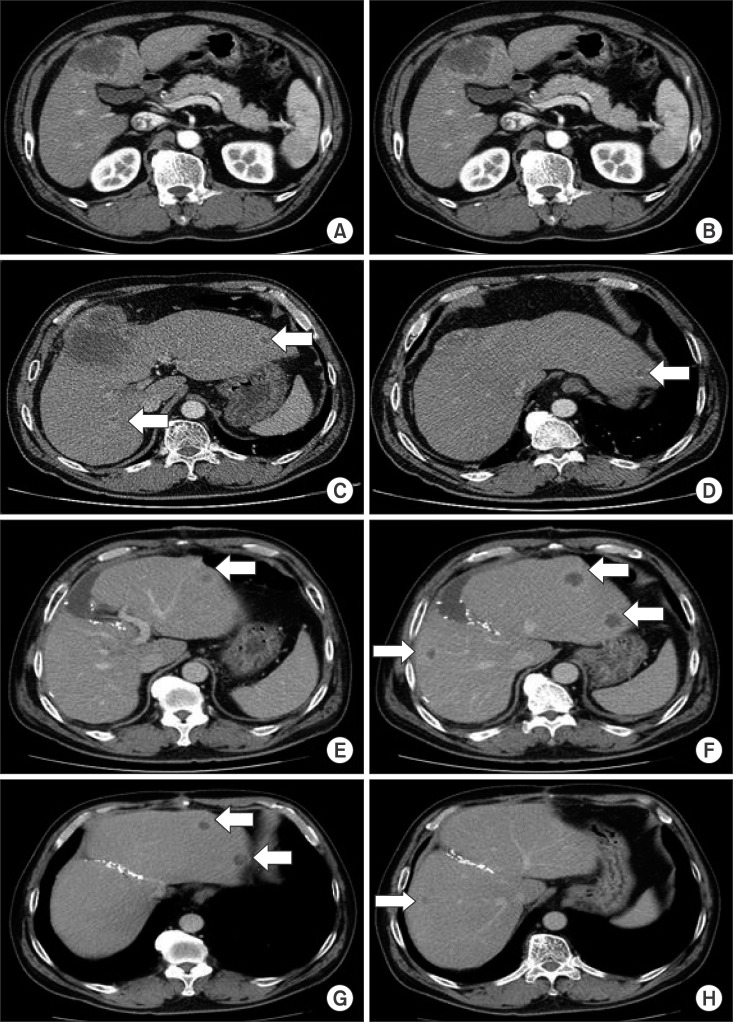
Fig. 2
Pathological findings. Gross appearance showed the multilobulated hemorrhagic mass with extracapsular mass extension (arrows) (A); Biphasic pleomorphic cells of cytotrophoblasts and syncytiotrophoblasts (arrow), and hemorrhage and necrosis (arrowhead) were observed (H&E stain, ×200) (B); Diffuse expression of β-hCG was identified in immunohistochemistry staining (×200) (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download