Abstract
Solitary fibrous tumor (SFT) of the liver is a rare entity and its presentation is usually delayed till they grow to a substantial size. Clinico-radiological features are non-specific, contributing to increase in the diagnostic dilemma. Definitive diagnosis of SFT is usually made based on the histological features and immunohistochemistry data of the resected specimen. In this case report, we describe the case of an elderly male who presented with a large mass in the left lobe of the liver with normal level of tumor markers and atypical radiological findings. The patient successfully underwent resection of the tumor and the diagnosis was confirmed on histopathology.
Solitary fibrous tumor (SFT) of the liver is a rare mesenchymal tumor. It is usually found in pleura and pelvis1 but can also occur in other unusual sites such as central nervous system, orbit, retroperitoneum, maxillary sinus, pancreas, and kidneys.2345 Histologically, they are characterized by proliferation of spindle cells, and immunohistochemically they are positive for CD34.6 Usually, SFTs are benign in nature, but 10–20% of them are reported to be malignant with a tendency to metastasize.7 Nonspecific clinico-radiological features of these tumors often delay the diagnosis as precise diagnosis can only be made based on histopathological data. Surgery is the best therapeutic modality with outcomes dependant on resectability.8
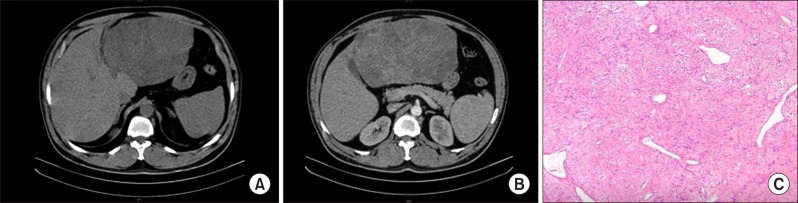
A 57-year old gentleman, with no co-morbidities, non-alcoholic, Hepatitis B/C negative, presented with a history of abdominal pain for the past 2 months. Examination revealed a large mass palpable in the epigastric region. Contrast-enhanced computed tomography (CT) scan showed a 19×18 cm-sized mass arising from the left lobe of liver/gastrohepatic omentum with heterogeneous enhancement in arterial and portal venous phase (Fig. 1). Gastro-duodenoscopy findings were normal. The level of tumor markers was within the normal limits (alpha-fetoprotein (AFP) 1.9 ng/ml, chorioembryonic antigen (CEA) 3.8 ng/ml, and carbohydrate antigen (CA) 19-9−5.6 U/ml). Liver functions were within the normal range. Based on the atypical imaging findings and the close proximity of the palpable mass to the stomach, biopsy of the lesion was done. The biopsy was suggestive of spindle cell tumor of low to intermediate malignant potential and immunohistochemistry (IHC) for gastrointestinal stromal tumor (GIST) was negative. Since the lesion was deemed resectable, surgery was planned. The patient underwent left lateral sectionectomy. Intraoperatively, a solitary large tumor arising from the segment III of the liver was identified with the stomach being normal and no evidence of distant metastasis. The postoperative course was uneventful.
Grossly, the tumor measured 18×17×13 cm in size, with a smooth external surface and extensive subcapsular hemorrhage. Cut section showed lobulated firm tissue, tan to white in color with foci of necrosis and hemorrhage. Focal areas showed a whorled pattern. Microscopic examination revealed haphazard arrangement of spindle cells and collagen bundles between the tumor cells with mild nuclear pleomorphism, and the mitotic count was 0-1/10 high power field (HPF) (Fig. 1). The parenchymal cut margin was free of tumor. On IHC, the tumor was positive for CD34, Bcl2, and Mic2 and negative for S100, SMA, cKit, and CD31, consistent with SFT. The patient has been advised regular observation and follow-up. There was no evidence of loco-regional or distant recurrence on follow-up for 3 months.
SFT primarily arising from the liver is an unusual occurrence, which makes diagnosis and treatment of this rare tumor challenging. SFT arising from the liver was first reported by Nevius and Friedman in 1959.9 These tumors are usually seen in an elderly population with a female preponderance.10
Clinical presentation of these tumors is usually non-specific and most of the patients are asymptomatic initially until the tumor attains a large size and presents with symptoms due to mass effect or cholestasis.11 The radiological findings of SFT are non-specific and it is hypothesized that other space-occupying lesions in the liver like hepatocellular carcinoma, sarcoma, and inflammatory pseudotumor can also have similar radiological features.12 Similarly, benign tumors cannot be reliably differentiated from malignant tumors due to overlapping features. On ultrasound, SFT typically appears hypoechoic but occasionally they are heterogeneous, likely due to areas of myxoid degeneration.13 On CT scan, they appear as a well-defined encapsulated mass with heterogeneous enhancement. On T1-weighted magnetic resonance images, these tumors appear as low or intermediate intensity lesions and on T2 sequence both hypointense and hyperintense areas are seen. These findings are suggestive but not diagnostic of hepatic SFT.1415
Definitive diagnosis of SFT can only be made based on the histological features of the resected specimen. Role of preoperative fine needle aspiration cytology or tru-cut biopsy is not clearly defined and usually not favored, as fine needle aspiration cytology can be misleading or inconclusive and biopsy can result in biopsy tract tumor seeding.111415 They are characterized by a pattern-less architecture formed by a combination of alternating hypocellular and hypercellular areas separated from each other by bands of hyalinized collagen and branching vessels.16 These characteristic histological features are useful in differentiating SFTs from other liver mesenchymal tumors.
IHC remains the most important tool for conclusive diagnosis of SFT. SFTs show consistent expression of CD34, with variable expression of CD99 and Bcl-2.17 IHC is of immense help in differentiating SFTs from other conditions like leiomyoma (SMA positive, CD34 negative), inflammatory pseudotumor (SMA positive, vimentin positive, and CD34 negative), fibrosarcoma (CD34 negative), and gastrointestinal stromal tumor (CD117 and CD34 positive).18 Although most of the hepatic SFTs are benign, they can exhibit malignant behavior at times, and histological features suggestive of aggressive behavior tendency include size >10 cm, cellular atypia, necrosis, and >4 mitoses/10 high porwer field.19
Complete surgical excision is the treatment of choice for hepatic SFT. If margin-negative resection is achieved, no further treatment is needed.19 For inoperable or incompletely resected tumors, liver-directed therapies in the form of trans-arterial chemoembolization may be offered, but there exists no robust evidence to support this modality.16 Until date, there exist no studies which describe long-term outcomes of these tumors. Therefore, follow up is advised as some of the patients with benign tumors on initial histological examination developed distant metastasis later.19
In conclusion, SFT arising from the liver is a rare entity. It should be considered as a differential diagnosis in liver lesions with atypical radiological findings. Complete surgical excision is advised for definitive diagnosis and to predict aggressiveness based on histological features. Long-term follow-up in patients with SFT is essential as the tumor biology remains unknown. Therefore, follow up is advised as some of the patients with benign tumors on initial histological examination developed distant metastasis later.
ACKNOWLEDGEMENTS
We would like to acknowledge the Department of Pathology, Tata Memorial Hospital, Mumbai, India for providing us the photomicrographs.
References
1. Bruzzone A, Varaldo M, Ferrarazzo C, Tunesi G, Mencoboni M. Solitary fibrous tumor. Rare Tumors. 2010; 2:e64. PMID: 21234256.

2. Cuello J, Brugés R. Malignant solitary fibrous tumor of the kidney: report of the first case managed with interferon. Case Rep Oncol Med. 2013; 2013:564980. PMID: 23401821.

3. Weon YC, Kim EY, Kim HJ, Byun HS, Park K, Kim JH. Intracranial solitary fibrous tumors: imaging findings in 6 consecutive patients. AJNR Am J Neuroradiol. 2007; 28:1466–1469. PMID: 17846192.

4. Chen H, Xiao CW, Wang T, Wu JS, Jiang CC, Qian J, et al. Orbital solitary fibrous tumor: a clinicopathologic study of ten cases with long-term follow-up. Acta Neurochir (Wien). 2012; 154:249–255. PMID: 22203231.

5. Miyamoto H, Molena DA, Schoeniger LO, Haodong Xu. Solitary fibrous tumor of the pancreas: a case report. Int J Surg Pathol. 2007; 15:311–314. PMID: 17652547.

6. Rao N, Colby TV, Falconieri G, Cohen H, Moran CA, Suster S. Intrapulmonary solitary fibrous tumors: clinicopathologic and immunohistochemical study of 24 cases. Am J Surg Pathol. 2013; 37:155–166. PMID: 23108019.
7. Tanaka M, Sawai H, Okada Y, Yamamoto M, Funahashi H, Hayakawa T, et al. Malignant solitary fibrous tumor originating from the peritoneum and review of the literature. Med Sci Monit. 2006; 12:CS95–CS98. PMID: 17006407.
8. Ji Y, Fan J, Xu Y, Zhou J, Zeng HY, Tan YS. Solitary fibrous tumor of the liver. Hepatobiliary Pancreat Dis Int. 2006; 5:151–153. PMID: 16481304.
9. Nevius DB, Friedman NB. Mesotheliomas and extraovarin thecomas with hypoglycemic and nephrotic syndromes. Cancer. 1959; 12:1263–1269. PMID: 14426759.
10. Patra S, Vij M, Venugopal K, Rela M. Hepatic solitary fibrous tumor: report of a rare case. Indian J Pathol Microbiol. 2012; 55:236–238. PMID: 22771653.

11. Fuksbrumer MS, Klimstra D, Panicek DM. Solitary fibrous tumor of the liver: imaging findings. AJR Am J Roentgenol. 2000; 175:1683–1687. PMID: 11090404.
12. Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997; 81:116–121. PMID: 9126139.
13. Liu Q, Liu J, Chen W, Mao S, Guo Y. Primary solitary fibrous tumors of liver: a case report and literature review. Diagn Pathol. 2013; 8:195. PMID: 24294990.

14. Peng L, Liu Y, Ai Y, Liu Z, He Y, Liu Q. Skull base metastases from a malignant solitary fibrous tumor of the liver. A case report and literature review. Diagn Pathol. 2011; 6:127. PMID: 22192457.

15. Sun K, Lu JJ, Teng XD, Ying LX, Wei JF. Solitary fibrous tumor of the liver: a case report. World J Surg Oncol. 2011; 9:37. PMID: 21443810.

16. Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998; 22:1501–1511. PMID: 9850176.
17. Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. World Health Organization classification of tumours of soft tissue and bone. Pathology and genetics. Lyon: IARC Press;2013. p. 80–82.
18. Changku J, Shaohua S, Zhicheng Z, Shusen Z. Solitary fibrous tumor of the liver: retrospective study of reported cases. Cancer Invest. 2006; 24:132–135. PMID: 16537181.

19. Soussan M, Felden A, Cyrta J, Morère JF, Douard R, Wind P. Case 198: solitary fibrous tumor of the liver. Radiology. 2013; 269:304–308. PMID: 24062563.

Fig. 1
Image findings: (A) Non-contrast computed tomography image showing a large mass in the left lobe of the liver in close proximity to stomach. (B) Heterogeneous enhancement on contrast image. (C) Typical morphological features of solitary fibrous tumor demonstrating arrangement of spindle cells in pattern-less architecture surrounding ectatic branching blood vessels with interspersed bands of hyalinized collagen (H&E stain, 10×).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download