Abstract
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare variant of bile duct tumors characterized by papillary growth within the bile duct lumen and recognized precursor of invasive carcinoma. IPNB was detected incidentally in a 60-year-old woman during check up. Radiologic images revealed a huge cystic mass with papillary projection and markedly dilated bile ducts. Biopsies revealed high-grade IPNB. Cholangioscopy detected a connection between the right posterior bile duct and cyst lumen with epithelial dysplasia of the bile duct. Right posterior sectional duct opened in the left hepatic duct. Consequently, right trisectionectomy and extrahepatic bile duct resection were conducted. Histological studies revealed intraductal papillary neoplasm with high-grade intraepithelial neoplasia (carcinoma in situ). IPNB patients without distant metastases are candidates for surgery and complete resection should be conducted to achieve long-term survival.
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare variant of bile duct tumors, characterized by papillary or villous growth within the bile duct lumen and is regarded as a biliary counterpart of intraductal papillary mucinous neoplasm (IPMN) of the pancreas.1 IPNB was recently defined in 2010 WHO classification as a distinct clinical and pathologic entity.2 IPNB is mostly found in the Far East nations where hepatolithiasis and clonorchiasis infections are endemic.3 Most patients are age 50–70.4 Common clinical signs of IPNB are abdominal pain, jaundice and cholangitis. The most common radiologic findings for IPNB are bile duct dilatation and intraductal masses.5 Here, we report surgical treatment of a huge asymptomatic IPNB.
A 60-year-old Japanese woman was admitted to our hospital due to a huge liver mass detected incidentally during the patient's routine respiratory examination diagnosed with bronchial asthma and obstructive pulmonary disease. She had no symptoms or past medical history of liver disease. She smoked 15 cigarettes per day and quit 2 months prior the operation. Laboratory values on admission were as follows: Total leucocytic count 5.17×103/cc, hemoglobin 13.4 g/dl, platelets count 274×109/L, aspartate aminotransferase 25 IU/L, alanine aminotransferase 28 IU/L, alkaline phosphatase 271 IU/L, gamma-glutamyl transpeptidase 38 IU/L, total and direct bilirubin 0.5 and 0.1 mg/dl, albumin 3.8 g/dl, α-fetoprotein (AFP) 4.1 ng/ml, carcinoembryonic antigen (CEA) 6.8 ng/ml and cancer antigen 19-9 (CA19-9) 0.6 U/ml.
Abdominal ultrasonography (US) and enhanced computed tomography (CT) revealed a large cystic mass with multiple internal papillary projections involving the right-hemiliver and left medialsection (Fig. 1). Magnetic resonance imaging (MRI) and magnetic resonance cholangiography (MRC) detected a large cystic lesion involving the right posterior bile ducts (Fig. 2).
Endoscopic retrograde cholangiogram (ERC) confirmed contrast filling of the cyst lumen (Fig. 3). On peroral cholangioscopy (POCS), a large amount of mucus and papillary proliferating epithelium was observed in the cyst cavity while the orifice of the posterior duct was invaded by the tumor. From the lower bile duct up to the hepatic hilum, right anterior segment bile duct and left hepatic duct endo-lumen had no abnormal findings (Fig. 4). Multiple biopsies were conducted from the cyst cavity. Based on above mentioned radiological and endovisual findings, a diagnosis of IPNB was reached. Biopsy results confirmed our diagnosis, as a high-grade IPNB. Positron emission tomography (PET) demonstrated malignant lesions with marginal dominance of FDG accumulation in the right lobe. Intra- and extra-hepatic metastases were not detected (Fig. 5). Three-dimensional liver CT scan demonstrated right, middle hepatic veins and right portal vein in contact with the tumor (Fig. 6).
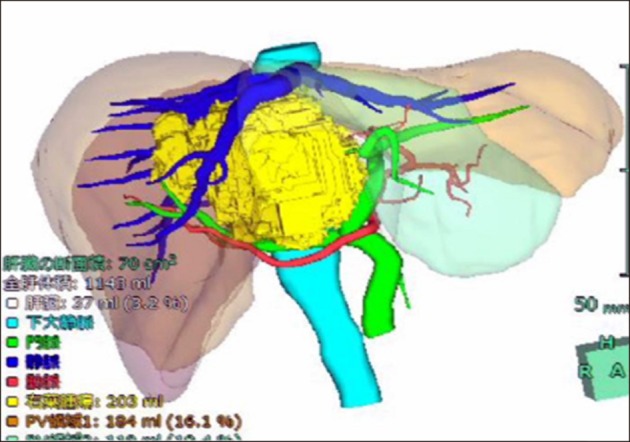
Based on findings, right trisectionectomy of liver with extrahepatic bile duct resection and reconstruction were planned. Liver volumetry was undertaken, total liver and the functional volume of the left lateral section were estimated at 1,143 ml (100%) and 303 ml (26.5%), respectively. So, we first conducted percutaneous transhepatic portal vein embolization (PTPE) to induce hypertrophy of the remnant liver. After 4 weeks, the estimated remnant liver volume increased to 434 ml (38%) (Figs. 7 and 8).
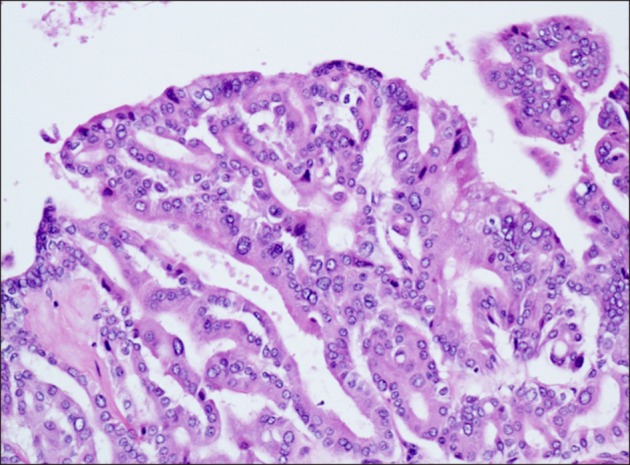
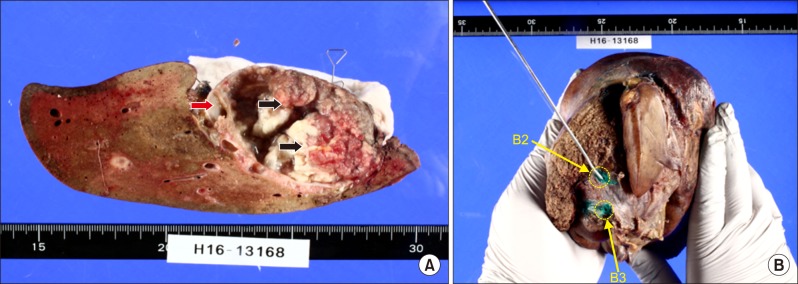
Four weeks after PTPE, the patient underwent surgery. Right trisectionectomy with extrahepatic bile duct resection and Roux-en-Y cholangiojejunostomy were conducted. Intraoperative frozen section confirmed cancer-free surgical margins, including segment II and III bile duct stumps. Segment II and III bile ducts were separately anastomosed to the ascending jejunal limb. Macroscopic examination of the resected specimen revealed a large mixed cystic-solid lesion with fibrous capsule and multiple papillary projections (Fig. 9). Histological studies revealed intraductal papillary neoplasm with high-grade intraepithelial neoplasia (carcinoma in situ), and no regional lymph node metastases (pTis pN0). Microscopic findings include prominent papillary proliferation with fibrovascular cores, and epithelial subtypes was a pancreatobiliary (Fig. 10). Immunohistochemical staining results were positive for CK7, MUC4, MUC5AC, MUC6, and negative for MUC1, MUC1, MUC2, CK20, p53. Ki-67 level was 20%. The patient's post-operative course was uneventful and was discharged from our institution on the 15th post-operative day. So far, 12 months post-operatively, the patient has no recurrence and appears healthy.
The INPB is a rare neoplasm of the liver, adopted as a distinct entity in the 2010 World Health Organization (WHO) classification.6 According to the WHO classification system, IPNB is defined as a cystic lesion lined with biliary, mucinous or oncocytic epithelium in papillary configurations without ovarian-like stroma.7 Recently, IPNB is a biliary counterpart of IPMN, because clinicopathologic and morphologic similarities.8
The most common presenting symptom of IPNB is intermittent abdominal pain, jaundice and acute cholangitis, however, up to 5% of patients may be asymptomatic.9 Approximately 30% of patients have history of hepatolithiasis and clonorchiasis infection.10 In our patient, the tumor was discovered incidentally with no history of hepatolithiasis or clonorchiasis infection. It is critical to diagnose IPNB correctly, because this tumor has malignant potential and shares similar radiological and clinical characteristics with other cystic liver lesions.2 IPNB can be detected in the extrahepatic and intrahepatic bile ducts.11 Radiologic findings of IPNB include bile duct dilatation and intraductal masses.12 The US, CT, MRI, ERC, POC and IDUS are useful for diagnosis of tumor extension, involvement of the bile duct and presence of mucin (Figs. 1, 2, 3, 4). Solid, irregular, thickened lesions in the cystic or dilated bile duct are radiologic findings strongly considered as evidence of malignancy.13 The POCS is available in a few centers. In our case, we conducted POCS to approach the bile duct directly, assess the extent of the tumor and conduct a biopsy for histological examination (Fig. 4). The POCS is useful in detecting superficial tumors spreading along the biliary epithelium, that is crucial to reach the right decision before surgery.14
Patients with IPNB should be treated, even if not malignant. Because, papillary tumors and mucin may lead to obstructive jaundice and cholangitis.10 In patients without distant metastasis, surgical intervention is the first choice of treatment including pancreaticoduodenectomy (31%), hemihepatectomy (28%), bile duct resection (18%), segmental liver resection (15%) or liver transplant (5%).15 It has been proposed that the curative resection of IPNB with a malignant potential has a favorable prognosis of 47.0–82.0% 5-year overall survival rate.161718 However, microscopic positive margin (R1 resection), invasive carcinoma, extraductal invasion, lymphovascular invasion, lymph node metastasis, tumor positivity for Mucin-1, and positivity for carcinoembryonic antigen (CEA) are poor prognostic factors with higher incidence of recurrence after surgical resection of IPNB.1819 Mucus production can occur after surgical resection, even if no malignant IPNB tissue remains. Therefore, biliary tract after management after surgical resection is paramount.
We report a rare case of IPNB in an asymptomatic patient. The US, CT, MR, MRCP, IDUS and POCS are useful in pre-operative diagnosis to assess tumor invasion depth and extension along the bile duct. IPNB patients without distant metastases are candidates for surgery. Complete resection should be conducted to achieve long-term survival.
References
1. Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004; 28:327–338. PMID: 15104295.
2. Nakanuma Y, Curabo MP, Franceschi S, Gores G, Paradis V, Sripa B. WHO classification of tumours of the digestive system. Lyon: IARC;2010. p. 217–224.
3. Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, et al. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014; 2014:459091. PMID: 24949206.

4. Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). 2009; 11:684–691. PMID: 20495637.

5. Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012; 107:118–125. PMID: 21946282.

6. Aoki S, Okayama Y, Kitajima Y, Hayashi K, Imai H, Okamoto T, et al. Intrahepatic biliary papilloma morphologically similar to biliary cystadenoma. J Gastroenterol Hepatol. 2005; 20:321–324. PMID: 15683443.

7. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC;2010. p. 3–8.
8. Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006; 44:1333–1343. PMID: 17058219.

9. Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013; 19:8595–8604. PMID: 24379576.

10. Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011; 35:512–521. PMID: 21412069.

11. Tan Y, Milikowski C, Toribio Y, Singer A, Rojas CP, Garcia-Buitrago MT. Intraductal papillary neoplasm of the bile ducts: a case report and literature review. World J Gastroenterol. 2015; 21:12498–12504. PMID: 26604656.

12. Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004; 100:783–793. PMID: 14770435.

13. Yoon KH, Ha HK, Kim CG, Roh BS, Yun KJ, Chae KM, et al. Malignant papillary neoplasms of the intrahepatic bile ducts: CT and histopathologic features. AJR Am J Roentgenol. 2000; 175:1135–1139. PMID: 11000178.
14. Sakai Y, Tsuyuguchi T, Ishihara T, Sugiyama H, Miyakawa K, Yasui S, et al. Usefulness of peroral cholangioscopy in preoperative diagnosis of intraductal papillary neoplasm of the bile duct. Hepatogastroenterology. 2010; 57:691–693. PMID: 21033211.
15. Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, et al. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2014; 27:73–86. PMID: 23828315.

16. Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008; 97:508–512. PMID: 18314868.

17. Hokuto D, Nomi T, Yasuda S, Yoshikawa T, Ishioka K, Yamada T, et al. Long-term observation and treatment of a widespread intraductal papillary neoplasm of the bile duct extending from the intrapancreatic bile duct to the bilateral intrahepatic bile duct: A case report. Int J Surg Case Rep. 2017; 38:166–171. PMID: 28763696.

18. Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, et al. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014; 21:176–185. PMID: 23908126.

19. Narita M, Endo B, Mizumoto Y, Matsusue R, Hata H, Yamaguchi T, et al. Multicentric recurrence of intraductal papillary neoplasms of bile duct in the remnant intrahepatic bile duct after curative resection. Int J Surg Case Rep. 2015; 12:123–127. PMID: 26070186.

Fig. 1
Preoperatoive imaging findings. (A) Abdominal ultrasonography (US) revealed multiple hyper-echoic solid lesions (arrows). (B) Enhanced computed tomography (CT) revealed a huge (10×7 cm) cystic tumor with surrounding high-density components (arrows) involving the right hemiliver and the left medial section.

Fig. 2
Non-enhanced magnetic resonance imaging (A) revealed huge solid mass in the right hemiliver and the left medial segment of the liver. Bile ducts were markly dilated. Suspicious communication between right posterior bile duct (arrow) and the cystic lesion. Magnetic resonance cholangiography (B) revealed a marked aneurysmal dilatation of the bile duct.
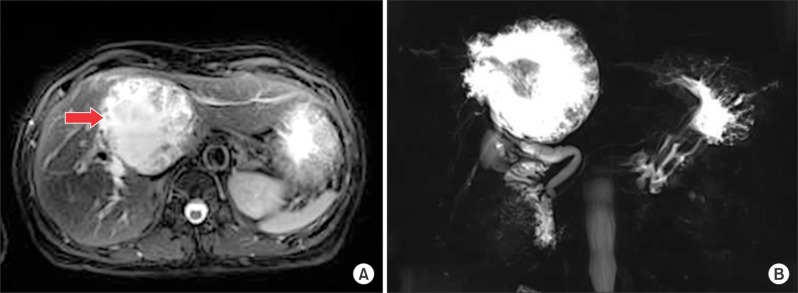
Fig. 3
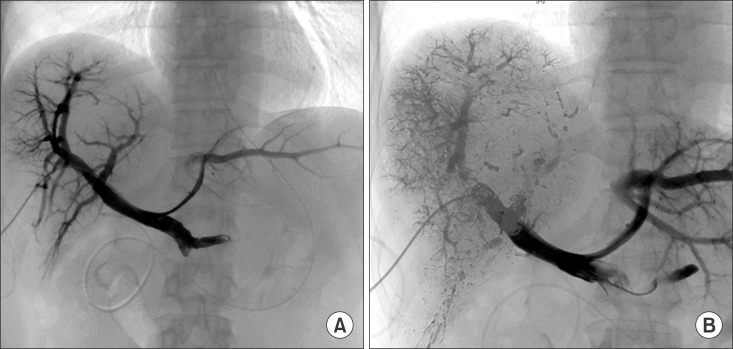
Endoscopic retrograde cholangiogram revealed communication between the proximal right hepatic duct and cyst.
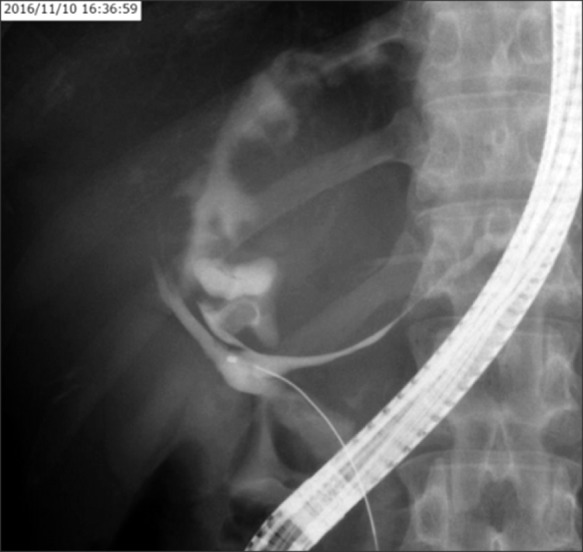
Fig. 4
Peroral cholangioscopy findings. Papilla Vater canulation (A). No abnormal finding on hepatic hilum (B). Tumor invading the right posterior bile duct (C). Biliary papillomatosis inside of the cyst (D).
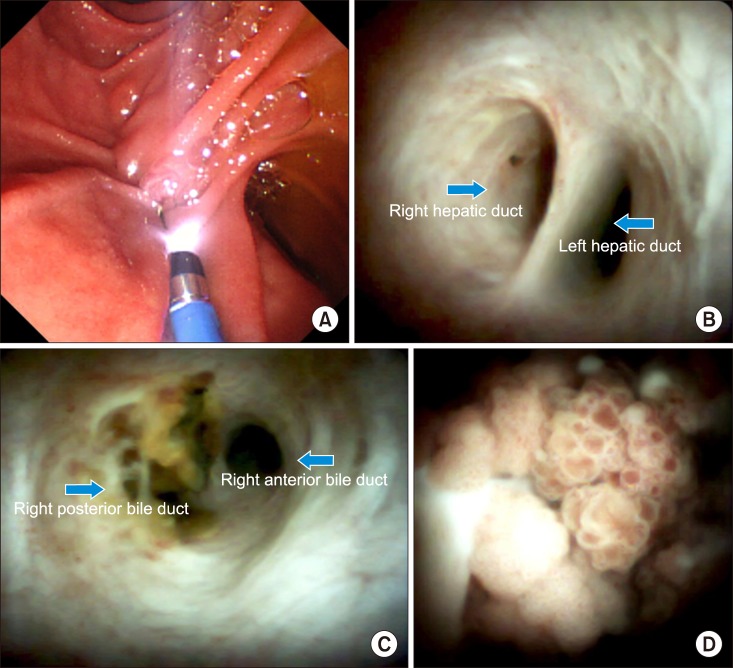
Fig. 5
Positron emission tomography-computed tomography scan revealed tumorous lesions with abnormal uptake and marginal dominance of FDG accumulation in the right lobe. Intrahepatic and extrahepatic metastasis were not detected.
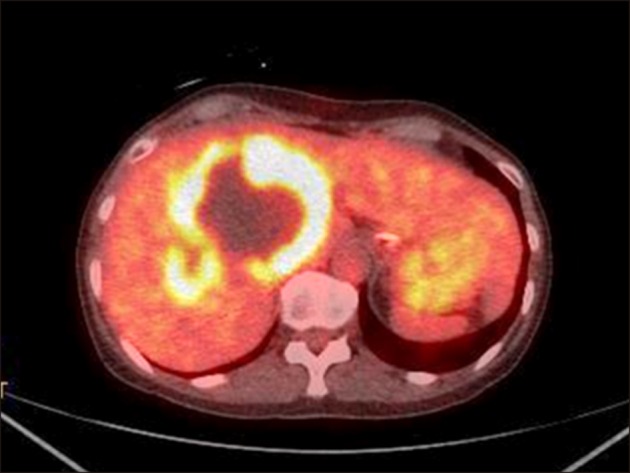
Fig. 6
Three-dimensional computed tomography image before percutaneous transhepatic portal vein embolization.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download